Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Allergy
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide causing a global pandemic. In this context, lung ultrasound (LUS) has played an important role due to its high diagnostic sensitivity, low costs, simplicity of execution and radiation safeness. Despite computed tomography (CT) being the imaging gold standard, lung ultrasound point of care exam is essential in every situation where CT is not readily available nor applicable.
- lung ultrasound
- COVID-19
- SARS-CoV-2
- pneumonia
- chest ultrasound
1. Lung Ultrasound in COVID-19 Pneumonia: Technique
LUS for COVID-19 can be performed by most ultrasound machines available. A lung preset is available for many recent machines but operators can adjust settings to provide good quality images [1].
The feasibility of a lung scan does not provide a predefined scheme for the evaluation and can be adapted to the needs of the clinician.
However, we suggest depth should be set between 8 and 10 cm and modulated according to the height of the patient and the thickness of his chest wall.
Focus should be positioned on the pleural line when available. It might be necessary to adjust the post processing settings to acquire the best image.
The ultrasound chest exploration should be systematic, starting from the anterior to the inferior areas along the intercostal spaces from medial to lateral.
If the patient is in the supine position, it is advisable to scan the anterior and lateral thorax dividing each hemithorax into 6 zones with diagnostic accuracy comparable to the 12-zone scanning scheme [2]. When the patient is in forced supine position, it may also be convenient to use an 8-zone scan scheme. In this approach, 4 zones, 2 anterior and 2 lateral, are examined for each hemithorax. The anterior zones are between the mid-clavicular line medially and the anterior axillary line laterally, while the lateral zones are between the anterior axillary line medially and the posterior axillary line laterally [3].
Regarding the ultrasound analysis of posterior regions of the thorax, which can be scanned if the patient is able to maintain the sitting position, the detection is performed along the paravertebral line, the scapular line and the posterior axillary line [4].
The ultrasound probe can be positioned transversely along the intercostal space or longitudinally perpendicular to the ribs. This last approach makes it possible to identify “the bat sign” (Figure 1), in which the bat’s wings are represented by the upper and lower ribs while the outline of the bat’s body is the pleural line [5].
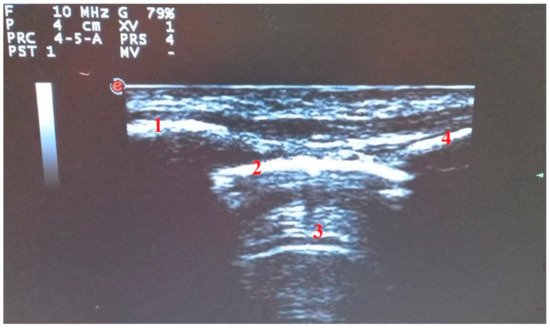
Figure 1. Longitudinal scan with linear probe: “the bat sign”. (1) Upper rib. (2) Pleural line. (3) A Lines. (4) Lower rib.
It is possible to choose any transducer: cardiac, convex, micro convex or linear. Usually, the convex probe is the most used because it allows a better visualization of the pleural line and subpleural space. Moreover, it guarantees a better evaluation of the diaphragmatic recess. Alternatively, using a phased array for both cardiac and lung ultrasound can reduce costs [5].
2. Lung Ultrasound in COVID-19 Pneumonia: Findings
LUS Assessment for SARS-CoV-2 Pneumonia is a Clinically Driven “Point of Care” Method.
LUS detects changes in the relationship between air and tissue at the surface of the lung [6].
Ultrasound cannot be transmitted through air and allows for air-filled lungs to create artifacts.
In a normally aerated lung, a pleural line appears as a hyperechoic horizontal line that moves synchronously with breaths [7]. Moreover, we recognize several hyperechoic lines parallel to the pleura named A lines. Those are repetition artifact that indicates a normally aerated parenchyma [8]. However, in patients with chronic obstructive pulmonary disease (COPD), atelectasis of the lung and asthma, the A lines are present and well represented in the presence of normal lung sliding, while in patients with pneumothorax, the only visible artifacts are the A lines in the absence of lung sliding [3]. The Z lines are also non-pathological vertical hyperechoic artifacts that originate from the pleural line but do not reach the border of the screen, do not move with the lung sliding and do not cancel the A lines. These features allow to differentiate the Z lines from the B lines, which are also hyperechoic lines and instead can take on pathological significance.
SARS-CoV-2 pneumonia is an interstitial pneumonia with a typically peripheral distribution.
It is characterized by progressive reduction of air-filled lungs and LUS acts as a densitometer. It detects changes in the abnormal ventilated parenchyma due to lung density increasing and air content decreasing [9].
The sonographic findings suggestive of SARS-CoV-2 pneumonia are B-line, fuse B-line (white lung), abnormalities of pleural line, small and large peripherical consolidations with or without bronchogram [10].
B lines are vertical hyperechoic artifacts originating from the pleural line and extending to the bottom of the image erasing the A lines [11].
B line move synchronously with lung sliding and are indicative of interstitial syndrome [12]. Cluster of B- lines are the ultrasound sign of the subpleural interlobular thickening. In the scanned fields, more than three B lines or their confluence, configuring the “white lung”, suggests an interstitial pneumonia SARS-CoV-2 and the number of B lines is associated with a greater severity of pulmonary involvement [13] (Figure 2).
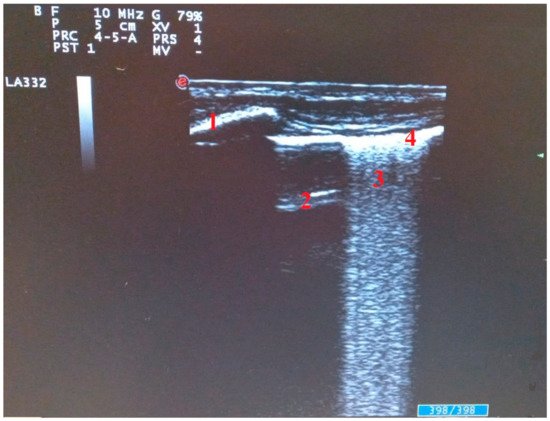
Figure 2. Ultrasound findings in SARS-CoV-2 pneumonia: (1) ribs, (2) A line, (3) cluster of B lines, (4) pleural line.
The Kerley B-lines visible on chest X-ray, which are the expression of the thickening of the interlobular septa in the interstitial syndrome, correlate with the ultrasound finding of B-lines in numbers greater than 3 per field [3]. In the initial phase, B lines have a focal distribution and there is a separation between them. As the disease progresses, B lines tend to merge and their distribution increases. In the resolution phase, they gradually disappear [4]. Consequently, ultrasound allows us to identify the different evolutionary stages of SARS-CoV-2 pneumonia [14].
The white lung is a multiple coalescent B line that completely occupies the lung field. It is due to alveolar de-aeration [11] and it correlates with ground glass on HRCT in SARS-CoV-2 pneumonia [11][13] (Figure 3).
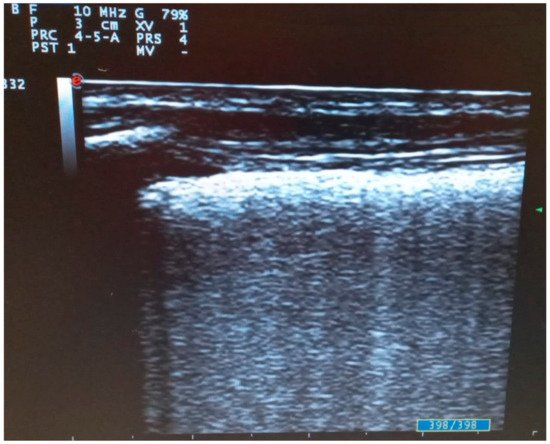
Figure 3. Transversal scan with linear probe of COVID-19 pneumonia: fused B lines configuring “white lung”.
Among vertical artifacts of SARS-CoV-2 pneumonia, “the light beam”, a bright vertical artifact that moves rapidly with sliding, correlates with the early phase “ground glass” observed on chest CT scan. It arises from a normal pleural line, goes within areas of normal pattern or with separate B lines and disappears quickly from the screen with an “on-off” effect [15].
Another suggestive ultrasound sign is abnormalities of the pleural line [16] (Figure 4). In the initial phase of the disease, small and diffuse irregular thickening of the pleural line appears. This artifact becomes more nodular in the appearance as the disease progresses, with areas of discontinuity that usually disappear if the disease progresses favorably [4].
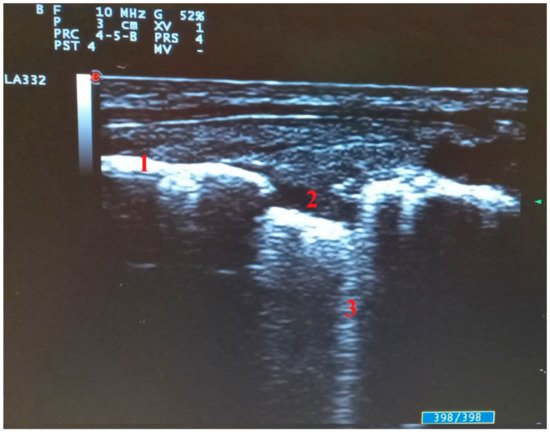
Figure 4. Abnormalities of pleural line in transversal scan: (1) pleural line, (2) pleural line interruption with subpleural consolidation, (3) single B line arising from subpleural consolidation.
In the subpleural space, consolidations can be found and appear on the ultrasound as areas of hepatization with irregular edges and air bronchogram [12].
Parenchymal consolidations increase with disease severity [17], while large consolidations with air bronchogram in the lower lobes raise the suspicion of bacterial superinfection [15] (Figure 5).
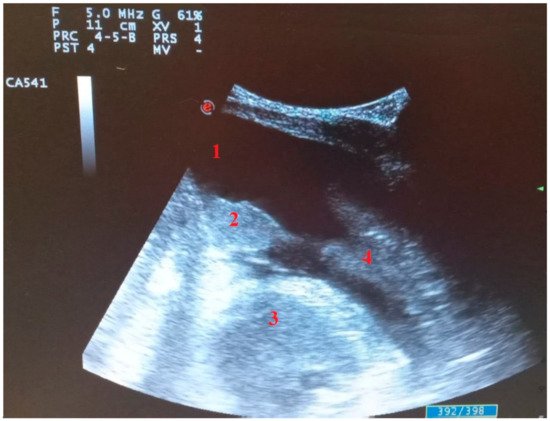
Figure 5. Longitudinal scan with convex probe in COVID-19 patient on mechanical ventilation with bacterial superinfection: (1) pleural effusion, (2) parenchymal consolidation without air bronchogram, (3) heart, (4) parenchymal consolidation with air bronchogram.
The pulmonary lesions are preferentially located in the posterior zone with bilateral distribution [18], while in severe forms it could progress to affect all lung fields [16], and have a patchy bilateral distribution of multiform cluster with sparing areas [19].
Complications of severe forms of SARS-CoV-2 pneumonia are pneumomediastinum and pneumothorax eventually associated with subcutaneous emphysema [20].
If pneumothorax (PNX) is present, there is no B line, lung pulse and sliding. If these absences are highlighted on the ultrasound scan, it is necessary to perform another scan along the middle axillary line. If the lung point is visible, the diagnosis of PNX will be probable with a sensitivity between 75% and 100% and a specificity between 94% and 100% [21].
If subcutaneous emphysema is present, on chest ultrasound it will be possible to visualize only B lines arising from the subcutaneous tissue and not from the pleural line that will not be visible as well as the subpleural space.
Scores
Many scores have been evaluated to assess thoracic ultrasound but the most commonly used is the lung ultrasound score (LUSS).
This score is widely used to assess patients in several clinical contexts. It allows evaluation of loss of aeration in the scanned area with a numeric outcome [22]. LUSS numerically describes the spread and progressive severity of pulmonary involvement. Moreover, numerical value is reduced in the successfully extubated patient. The 12 scanning zones are evaluated and a score from 0 to 3 is assigned for each one: 0 is assigned to a normal ultrasound pattern, 1 to the presence of the B lines, 2 to the white lung and 3 to consolidations [23]. The total score goes from 0 to 36 and it correlates with increasing lung involvement severity.
Correlation between LUSS and lung weight has been extensively demonstrated [24] and it could be a useful tool for assessing severity of SARS-CoV-2 pneumonia and monitoring the progression of lung involvement [25].
LUSS evaluation for SARS-CoV-2 has been shown to be a valuable choice for ICU patients [23][25]. Furthermore SARS-CoV-2 pneumonia and SARS-CoV-2 ARDS have specific features. In fact, posterior consolidations are preponderant in SARS-CoV-2 pneumonia and this feature weakens LUSS accuracy [26].
A specific ultrasound approach for SARS-CoV-2 pneumonia has been proposed since 2020 [27].
This new score evaluates the scan of seven areas in each hemithorax (three posterior, two lateral, two anterior). The scanned are located:
- -
-
On the paravertebral line upon the curtain sign.
- -
-
On the para-vertebral line at the inferior angle of the scapula.
- -
-
On the para-vertebral line at the spine of the scapula.
- -
-
On the mid-axillary line below the inter-nipple line.
- -
-
On the mid-axillary line above the inter-nipple line.
- -
-
On the mid-clavicular line below the inter-nipple line.
- -
-
On the mid-clavicular line above the inter-nipple line.
This proposal needs two skilled operators and pocket devices to perform ultrasound. The aim is to minimize risk to health workers operators and reduce the ultrasound operator-dependance. The limits of this score are shortage of skilled operators and the small number of pocket devices readily available.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11122381
References
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic ultrasonography: A narrative review. Intensive Care Med. 2019, 45, 1200–1211.
- Heldeweg, M.L.A.; Lieveld, A.W.E.; De Grooth, H.J.; Heunks, L.M.A.; Pieter, R.; Tuinman, P.R. ALIFE study group. Determining the optimal number of lung ultrasound zones to monitor COVID-19 patients: Can we keep it ultra-short and ultra-simple? Intensive Care Med. 2021, 47, 1041–1043.
- Di Serafino, M.; Notaro, M.; Rea, G.; Iacobellis, F.; Paoli, V.D.; Acampora, C.; Ianniello, S.; Brunese, L.; Romano, L.; Vallone, G. The lung ultrasound: Facts or artifacts? In the era of COVID-19 outbreak. Radiol. Med. 2020, 125, 738–753.
- Pérez Pallarés, J.; Flandes Aldeyturriaga, J.; Cases Viedma, E.; Cordovilla Pérez, R. SEPAR-AEER Consensus Recommendations on the Usefulness of the Thoracic Ultrasound in the Management of the Patient with Suspected or Confirmed Infection with COVID-19. Arch. Bronconeumol. 2020, 56 (Suppl. 2), 27–30.
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25.
- Soldati, G.; Smargiassi, A.; RInchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J. Ultrasound Med. 2020, 39, 1459–1462.
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imaging 2018, 11, 1692–1705.
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1.
- Stefanidis, K.; Dimopoulos, S.; Nanas, S. Basic principles and current applications of lung ultrasonography in the intensive care unit. Respirology 2011, 16, 249–256.
- Guarracino, F.; Vetrugno, L.; Forfori, F.; Bove, T. Lung, heart, vascular, and diaphragm ultrasound examination of COVID-19 patients: A comprehensive approach. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1866–1874.
- Boccatonda, A.; Cocco, G.; Ianniello, E.; Montanari, M.; D’Ardes, D.; Borghi, C.; Giostra, F.; Copetti, R.; Schiavone, C. One year of SARS-CoV-2 and lung ultrasound: What has been learned and future perspectives. J. Ultrasound 2021, 24, 115–123.
- Jackson, K.; Butler, R.; Aujayeb, A. Lung ultrasound in the COVID-19 pandemic. Postgrad. Med. J. 2021, 97, 34–39.
- Soccorsa, S.; Boccatonda, A.; Montanari, M.; Spampinato, M.; D’Ardes, D.; Cocco, G.; Accogli, E.; Cipollone, F.; Schiavone, C. Thoracic ultrasound and SARS-COVID-19: A pictorial essay. J. Ultrasound 2020, 23, 217–221.
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. On Lung Ultrasound Patterns Specificity in the Management of COVID-19 Patients. J. Ultrasound Med. 2020, 39, 2283–2284.
- Volpicelli, G.; Lamorte, A.; Villén, T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. 2020, 46, 1445–1448.
- Changyang, X.; Qiaoying, L.; Hong, D.; Wenzhen, K.; Jianqi, L.; Lijun, Y. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit. Care 2020, 24, 174.
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 75, 1096–1104.
- Yang, Y.; Huang, Y.; Gao, F.; Yuan, L.; Wang, Z. Lung ultrasonography versus chest CT in COVID-19 pneumonia: A two-centered retrospective comparison study from China. Intensive Care Med. 2020, 46, 1761–1763.
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020, 12, 22.
- Elhakim, T.S.; Abdul, H.S.; Romero, C.P.; Rodriguez-Fuentes, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep. 2020, 13, e239489.
- Wimalasena, Y.; Kocierz, L.; Strong, D.; Watterson, J.; Burns, B. Lung ultrasound: A useful tool in the assessment of the dyspnoeic patient in the emergency department. Fact or fiction? Emerg. Med. J. 2018, 35, 258–266.
- Chiumello, D.; Mongodi, S.; Algieri, I.; Vergani, G.L.; Orlando, A.; Via, G.; Crimella, F.; Cressoni, M.; Mojoli, F. Assessment of Lung Aeration and Recruitment by CT Scan and Ultrasound in Acute Respiratory Distress Syndrome Patients. Crit. Care Med. 2018, 46, 1761–1768.
- Dargent, A.; Chatelain, E.; Kreitmann, L.; Quenot, J.P.; Cour, M.; Argaud, L.; COVID-LUS Study Group. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS ONE 2020, 15, e0236312.
- Zhao, Z.; Jiang, L.; Xi, X.; Jiang, Q.; Zhu, B.; Wang, M.; Xing, J.; Zhang, D. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm. Med. 2015, 15, 98.
- Vetrugno, L.; Bove, T.; Orso, D.; Barbariol, F.; Bassi, F.; Boero, E.; Ferrari, G.; Kong, R. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography 2020, 37, 625–627.
- Pisani, L.; Vercesi, V.; Van Tongeren, P.S.I.; Lagrand, W.; Leopold, S.; Huson, M.; Henwood, P.; Walden, A.; Smit, M.; Riviello, E.; et al. The diagnostic accuracy for ARDS of global versus regional lung ultrasound scores—A post hoc analysis of an observational study in invasively ventilated ICU patients. Intensive Care Med. Exp. 2019, 7 (Suppl. 1), 44.
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J. Ultrasound Med. 2020, 39, 1413–1419.
This entry is offline, you can click here to edit this entry!
