Non-alcoholic fatty liver disease (NAFLD) is the most common form of liver disease all over the world due to the obesity pandemic; currently, therapeutic options for NAFLD are scarce, except for diet recommendations and physical activity. NAFLD is characterized by excessive accumulation of fat deposits (>5%) in the liver with subsequent inflammation and fibrosis. Studies in the literature show that insulin resistance (IR) may be considered as the key mechanism in the onset and progression of NAFLD. Using natural products, for example, spirulina, oleuropein, garlic, berberine, resveratrol, curcumin, ginseng, glycyrrhizin, coffee, cocoa powder, epigallocatechin-3-gallate, and bromelain, as an alternative approach in the treatment of NAFLD has drawn growing attention among physicians.
- natural products
- nutraceutics
- NAFLD
- animal models
- RCTs
1. Introduction
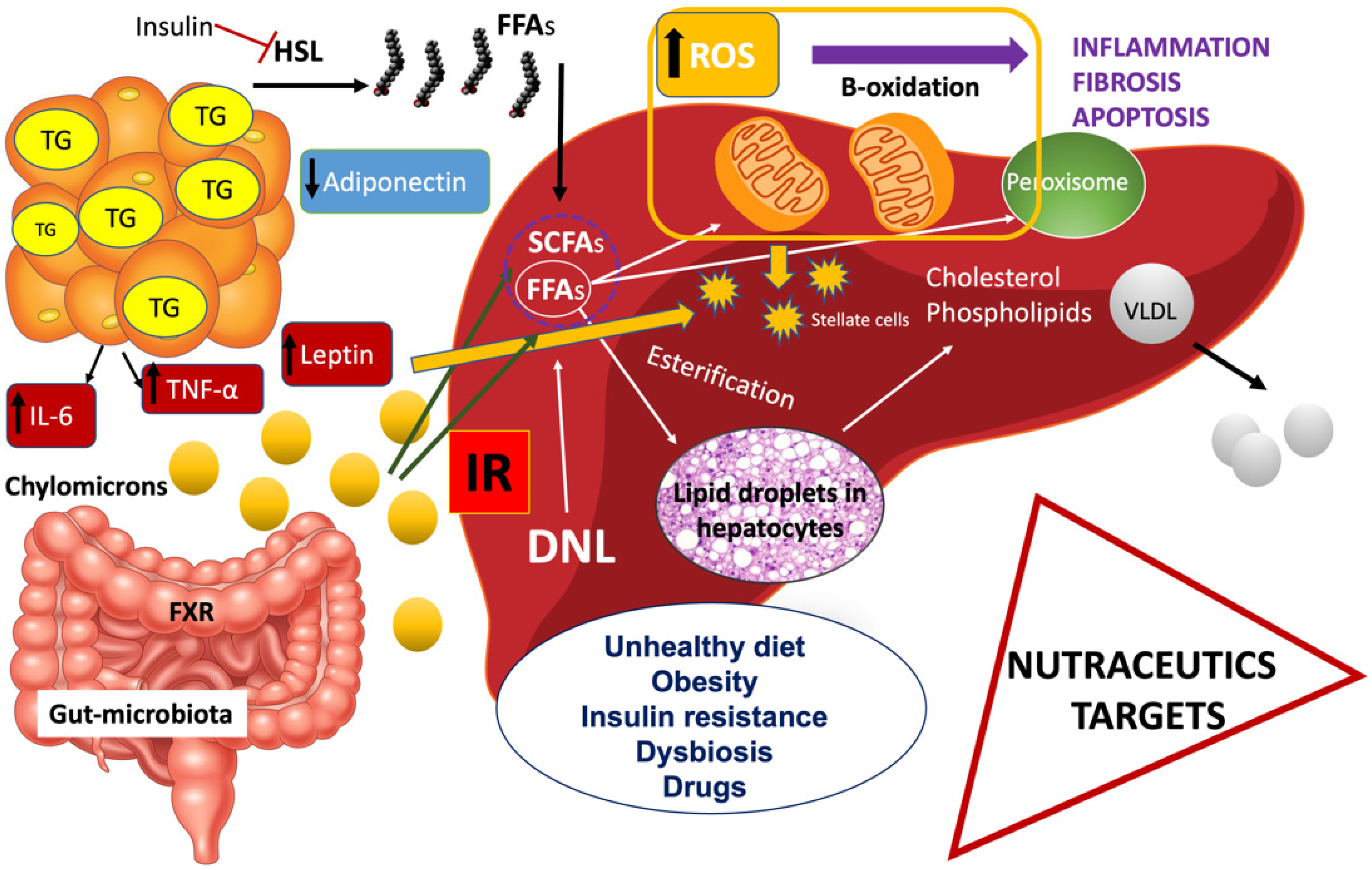
2. Clinical Trials and Studies in Animal Models
2.1. Alga Spirulina Maxima
2.2. Olive Oil
2.3. Garlic
2.4. Berberine
2.5. Resveratrol
2.6. Curcumin
2.7. Ginseng
2.8. Glycyrrhizin
2.9. Coffee
2.10. Cocoa Powder
2.11. Green Tea
2.12. Bromelain
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413424
References
- Jonas, W.; Schürmann, A. Genetic and epigenetic factors determining NAFLD risk. Mol. Metab. 2021, 50, 33160101.
- Arciello, M.; Gori, M.; Maggio, R.; Barbaro, B.; Tarocchi, M.; Galli, A.; Balsano, C. Environmental pollution: A tangible risk for NAFLD pathogenesis. Int. J. Mol. Sci. 2013, 14, 22052–22066.
- Zein, C.O.; Unalp, A.; Colvin, R.; Liu, Y.C.; McCullough, A.J. Nonalcoholic Steatohepatitis Clinical Research Network. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J. Hepatol. 2011, 54, 753–759.
- Tarantino, G.; Citro, V.; Capone, D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 2019, 9, 15.
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 15, 1343–1351.
- Seppälä-Lindroos, A.; Vehkavaara, S.; Häkkinen, A.-M.; Goto, T.; Westerbacka, J.; Sovijärvi, A.; Halavaara, J.; Yki-Järvinen, H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002, 87, 3023–3028.
- Du Plessis, J.; Van Pelt, J.; Korf, H.; Mathieu, C.; Van der Schueren, B.; Lannoo, M.; Oyen, T.; Topal, B.; Fetter, G.; Nayler, S.; et al. Association of adipose tissue inflammation with histological severity of non-alcoholic fatty liver disease. Gastroenterology 2015, 149, 635–648.e14.
- Shimabukuro, M. Leptin Resistance and Lipolysis of White Adipose Tissue: An Implication to Ectopic Fat Disposition and Its Consequences. J. Atheroscler. Thromb. 2017, 24, 1088–1089.
- Fruebis, J.; Tsao, T.S.; Javorschi, S.; EbbetsReed, D.; Erickson, M.R.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010.
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460.
- Boded, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10.
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379.
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Fousteris, A.A.; Kotsiris, D.A.; Lampadakis, I.M.; Ganotakis, E.S. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: A prospective pilot study. Ann. Gastroenterol. 2014, 27, 387–394.
- Ferreira-Hermosillo, A.; Torres-Duran, P.V.; Juarez-Oropeza, M.A. Hepatoprotective effects of Spirulina maxima in patients with non-alcoholic fatty liver disease: A case series. J. Med. Case Rep. 2010, 4, 103.
- Abenavoli, L.; Milanović, M.; Milić, N.; Luzza, F.; Giuffrè, A.M. Olive oil antioxidants and non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 739–749.
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161.
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2018, 72, 8–17.
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: A double-blind randomised controlled clinical trial. Br. J. Nutr. 2020, 124, 450–456.
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428.
- Maeda, T.; Miki, S.; Morihara, N.; Kagawa, Y. Aged garlic extract ameliorates fatty liver and insulin resistance and improves the gut microbiota profile in a mouse model of insulin resistance. Exp. Ther. Med. 2019, 18, 857–866.
- Neag, M.A.; Mocan, A.; Echeverrìa, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic and Renal Disorders. Front. Pharmacol. 2018, 9, 557.
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. Randomized Controlled Trial. PLoS ONE 2015, 10, e0134172.
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232.
- Guo, H.; Zhong, R.; Liu, Y.; Jiang, X.; Tang, X.; Li, Z.; Xia, M.; Ling, W. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of nonalcoholic fatty liver disease. Nutrients 2014, 30, 198–203.
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendia, L.E.; Sahebkar, A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: A randomized controlled trial. Drug Res. 2017, 67, 244–251.
- Hong, M.; Lee, Y.H.; Kim, S.; Suk, K.T.; Bang, C.S.; Yoon, J.H.; Baik, G.H.; Kim, D.J.; Kim, M. Anti-inflammatory and antifatigue effect of korean red ginseng in patients with nonalcoholic fatty liver disease. J. Ginseng Res. 2016, 40, 203–210.
- Hajiaghamohammadi, A.A.; Ziaee, A.; Samimi, R. The efficacy of licorice root extract in decreasing transaminase activities in non-alcoholic fatty liver disease: A randomized controlled clinical trial. Phytother. Res. 2012, 26, 1381–1384.
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review. Phytother Res. 2017, 31, 1635–1650.
- Mikolasevic, I.; Domislovic, V.; Filipec Kanizaj, T.; Radic-Kristo, D.; Krznaric, Z.; Milovanovic, T.; Stimac, D. Relationship between coffee consumption, sleep duration and smoking status with elastographic parameters of liver steatosis and fibrosis; controlled attenuation parameter and liver stiffness measurements. Int. J. Clin. Pract. 2020, 75, e13770.
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012, 55, 429–436.
- Parsaeyan, N.; Mozaffari-Khosravi, H.; Absalan, A.; Mozayan, M.R. Beneficial effects of cocoa on lipid peroxidation and inflammatory markers in type 2 diabetic patients and investigation of probable interactions of cocoa active ingredients with prostaglandin synthase-2 (PTGS-2/COX-2) using virtual analysis. J. Diabetes Metab. Disord. 2014, 13, 30.
- McShea, A.; Ramiro-Puig, E.; Munro, S.B.; Casadesus, G.; Castell, M.; Smith, M.A. Clinical benefit and preservation of flavonoids in dark chocolate manufacturing. Nutr. Rev. 2008, 66, 630–641.
- Satapati, S.; Kucejova, B.; Duarte, J.A.; Fletcher, J.A.; Reynolds, L.; Sunny, N.E.; He, T.; Nair, L.A.; Livingston, K.A.; Fu, X.; et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Investig. 2015, 125, 4447–4462.
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208.
- Kobayashi, M.; Kawano, T.; Ukawa, Y.; Sagesaka, Y.M.; Fukuhara, Y. Green tea beverages enriched with catechins with a galloyl moiety reduce body fat in moderately obese adults: A randomized double-blind placebo- controlled trial. Food Funct. 2016, 7, 498.
- Naito, Y.; Uschiroda, C.; Mizushima, K.; Inoue, R.; Yasukawa, Z.; Abe, A.; Takagi, T. Epigallocatechin-3-gallate (EGCG) attenuates non-alcoholic fatty liver disease via modulating the interaction between gut microbiota and bile acids. J. Clin. Biochem. Nutr. 2020, 67, 2–9.
- Hu, P.A.; Chen, C.H.; Guo, B.C.; Kou, Y.R.; Lee, T.S. Bromelain Confers Protection against the Non-Alcoholic Fatty Liver Disease in Male C57bl/6 Mice. Nutrients 2020, 12, 1458.
