Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
食用全谷物对糖尿病患者的葡萄糖代谢有益。
- whole grains
- diabetes mellitus
- meta-analysis
- randomized controlled trials
一、介绍
Diabetes mellitus (DM), which is usually characterized by an absolute or relative deficiency of serum insulin concentrations, is one of most serious metabolic diseases with a sharply increasing incidence globally. It is estimated that there were about 537 million diabetics among the population aged from 20–79 worldwide in 2021, and this number is expected to reach 784 million in 2045 [1]. According to a report from the World Health Organization, since 1980, the global prevalence of DM has almost doubled from 4.7% to 8.5% in the adult population [2]. In China, there is one diabetic patient in each 12 adults; worse, the estimated prevalence of prediabetes had risen to 35.7% in 2017 [3], indicating a serious economic burden in the future.
DM is caused by both genetic and environmental factors. Findings from mechanistic studies suggest that DM can lead to abnormal changes including metabolic profiles, energy production, redox status, and extracellular matrix remodelling [4], eventually resulting in atherosclerotic cardiovascular diseases. It is reported that adults with diabetes have a 2–4 times higher cardiovascular risk than adults without diabetes [5]. A meta-analysis performed by Einarson et al. indicated that about half of the deaths in patients with type 2 diabetes mellitus (T2DM) comprise cardiovascular diseases (CVDs) [6]. Therefore, researchers believe that poor management of DM patients can increase the possibility of suffering from reduced life quality and expectancy.
Previous studies have provided credible evidence for the key role of diet in the prevention of DM [7,8]. Whole grains are a group of cereal foods in which the endosperm, germ, and bran are intact. They are also a good source of dietary fibres, vitamins, antioxidants, and phytochemicals [9], such as phenolic compounds (including ferulic acids and cinnamic), beta-glucan, and lignans, which have been reported to play a protective role in many metabolic diseases, such as T2DM, obesity and CVDs [10,11]. The gut microbiota is referred to as a community of more than 1014 bacteria residing in the human intestine, and emerging discoveries have suggested that dysbiosis of the gut microbiota can result in many age-related and lifestyle diseases such as diabetes, obesity, inflammation, and CVDs [12,13,14]. A study by Zhao et al. suggested that T2DM could be alleviated by dietary fibres, which can be fermented by the gut microbiota and produce the beneficial metabolites of short-chain fatty acids (SCFAs) [15]. Indeed, a 6-week randomized controlled parallel-design trial conducted in postmenopausal women indicted that whole grain consumption led to an increased abundance of Lachnospira and the level of stool acetate; furthermore, Lachnospira was positively associated with acetate [16]. Similarly, a cross-sectional study has proposed significant associations between circulating SCFA, particularly acetate and propionate, and peripheral insulin sensitivity, whole body lipolysis and glucagon-like peptide-1 (GLP-1) concentrations [17]. In an in vivo study, Lappi et al. and Nilsson et al. also found that the consumption of barley kernel-based bread or wholegrain rye bread resulted in improved markers of glucose metabolism with increased serum SCFAs concentrations in healthy individuals [18,19]. A meta-analysis of cohort studies has established an inverse association between whole grain intake and the risk of T2DM [20]. However, the results are inconsistent in clinical trials. Li et al. found that replacing a healthy diet (low-fat and high-fibre diet) with 100 g of oats for 30 days leaded to a significant improvement in fast plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and homeostasis model assessment of insulin resistance (HOMA-IR) in overweight diabetics [21]. On the other hand, a study by McGeoch et al. showed no significant differences in terms of glycaemic control following intervention with the oat-enriched diet [22]. This discrepancy may be a consequence of differences in population selection and the type and amount of whole grain production consumed. Given the inconsistent results from clinical randomized controlled trials and the absence of any systematic reviews and meta-analyses, it is necessary to perform the present study.
2. Effects of Whole Grain Consumption on Parameters Involve Glycaemic Control
Since about 1980, because of shifts in dietary patterns and a more sedentary lifestyle, the global prevalence of DM has nearly doubled. Raised blood glucose, a common effect of uncontrolled diabetes, may, over time, lead to serious damage to the heart, blood vessels, eyes, kidneys, and nerves. However, results from a meta-analysis of prospective studies indicated that whole grain may have beneficial effects on glucose modulation [8]. These findings provide us with a new strategy for managing DM and avoiding undesirable effects caused by drug treatments.
2.1. Fast Plasma GLUCOSE Concentrations
The pooled effects of whole grain consumption on FPG concentrations were reported by fourteen studies in twelve articles, which showed a significant decrease in FPG concentrations (WMD = −0.51 mmol/L, 95% CI: −0.73, −0.28, Figure 1A) in subjects who consumed whole grain than those in a control group, while substantial evidence of heterogeneity was found between the studies (Figure 1A). Of note, the results of subgroup analysis by national economic level indicated no heterogeneity within studies performed in developed countries; moreover, no significant difference was observed between groups (WMD = −0.03 mmol/L, 95% CI: −0.11, 0.04).
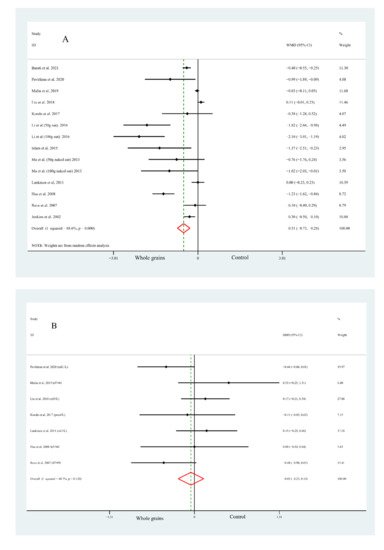
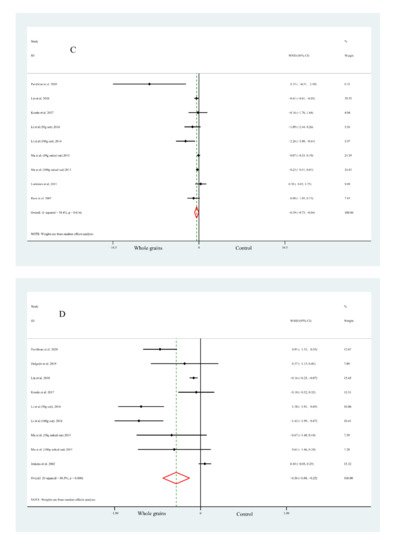
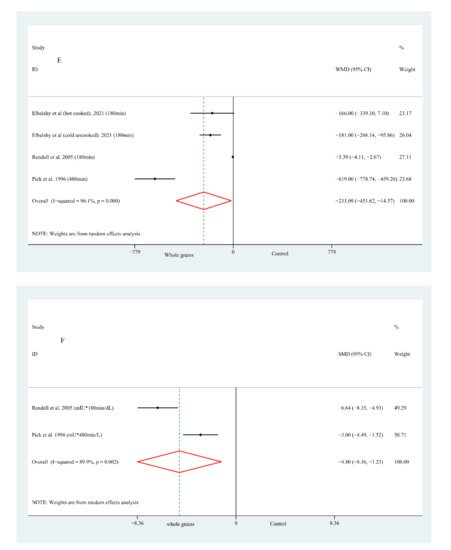
Figure 1. Forest plot of the effects of whole grain consumption on FPG (A), FPI (B), HOMA-IR (C), HbA1c (D), G-iAUC (E) and I-iAUC (F) in diabetic patients.
2.2. Fast Plasma Insulin Concentrations
The present meta-analysis consisting of seven studies demonstrated that, compared with the control group, the concentrations of FPI were not significantly changed by whole grain consumption (SMD = −0.05, 95% CI: −0.25, 0.14, Figure 1B), with a moderate heterogeneity (Figure 1B). In addition, researchers found a lower heterogeneity when it comes to the subgroup analysis by matching foods (0% for standard diet and 34.7% for others), which suggested that the controlled foods may play an important role in the evaluated outcomes.
2.3. Homeostasis Model Assessment of Insulin Resistance
Data on the effects of whole grain consumption on HOMA-IR were calculated by nine studies based on seven articles. As is shown in Figure 1, whole grain consumption led to a significant reduction in HOMA-IR (WMD = −0.39 μU × mol/L2, 95% CI: −0.73, −0.04, Figure 2C), though with obvious heterogeneity (Figure 1C). Further subgroup analysis suggested that the pooled effect concluded from three studies conducted in developed countries was not significant and with low heterogeneity, which showed some similarities to FPG.
2.4. Glycosylated Haemoglobin
Overall, nine effect sizes from seven clinical trials were eligible to assess the pooled effects of whole grain consumption on HbA1c in diabetic patients. A notable decrease in pooled effect on HbA1c was observed after whole grain consumption (WMD = −0.56%, 95% CI: −0.88, −0.25, Figure 1D); however, there was still a serious heterogeneity before subgroup analysis (Figure 1D). Consequently, subgroup analysis was conducted to explore the potential sources of heterogeneity. Interestingly, when researchers focused on short-term studies (<8 weeks) or those conducted in developed countries, the results became non-significant.
2.5. Glucose/Insulin Incremental Area under the Curve
Fewer studies had reported the effects of whole grain consumption on the G-iAUC and I-iAUC. As shown in Figure 1, the pooled WMD for whole grain on the G-iAUC was −233.09 min × mmol/L (95% CI: −451.62, −14.57; Figure 1E) and with high heterogeneity (Figure 2E); on the other hand, there were only two studies that reported data on I-iAUC, and the pooled SMD value was −4.80 (95% CI: −8.36, −1.23; Figure 1F). A serious heterogeneity (Figure 1F) was observed as well. Since the relevant studies were scarce, it is unnecessary to conduct any further subgroup analysis
3. Discussion
To the best of The knowledge, the present study is the first systematic review and meta-analysis of randomized controlled trials to provide evidence for the effects of whole grain consumption on glucose metabolism in diabetic patients. The results, concluded from 16 studies involving 1068 subjects, indicated that compared with the control group, whole grain consumption could significantly decrease the concentration of FPG, HOMA-IR, and HbA1c, while no significant difference was observed in FPI. Additionally, significant decreases in G-iAUC and I-iAUC after whole grain intake were also observed, although the relevant studies were scarce. Different matching foods and national economic levels may explain some unknown heterogeneity.
Recently, the results from a meta-analysis of prospective cohort studies suggested that compared with a low daily intake of dietary fibre, a 35 g daily intake could make an absolute reduction of 14 fewer deaths for each 1000 participants [41]. Indeed, a pilot study conducted by Khalil et al. suggested that a 12-week whole grain plant-based diet significantly decreased the level of FPG and HbA1c in newly diagnosed diabetics [42]. Consistently, The results also showed notable improvements in FPG and HbA1c after whole grain intake, in which HbA1c is more commonly used to diagnose and identify those at higher risk of developing diabetes in the future [43]. One proposed mechanism for those effects is that fibres contained in whole grain could increase the viscosity of intestinal content, which results in slowing down the absorption of glucose and eventually delaying the gastric emptying rate [44]. In an in vitro study, Abbasi et al. found that oat-derived β-glucan could significantly reduce the uptake of glucose in non-transformed rat small intestine epithelial cells by downregulating the expressions of glucose transporters sodium–glucose-linked transport protein 1 and glucose transporter 2 [45]. Moreover, the evidence for the relationship between the gut microbiota and health has been well-established, which drives us to consider the extensive role of the gut microbiota in the glucose metabolism. A study by Qin et al. indicated that the gut microbiota of patients with T2DM is mainly characterized by a decreased abundance of butyrate-producing bacteria, namely Roseburia and Faecalibacterium prauznitzii, compared with healthy subjects [46]. Furthermore, the gut microbiota was reported to be able to deconjugate bile acid, splitting off taurine or glycine molecules. Subsequently, the unconjugated bile acids can be dehydroxylated by specific strains from the Clostridium genus in the large intestine, which can act as better ligands for the farnesoid X receptor (FXR) and G protein-coupled (such as TGR5) receptors [47]. TGR5 activation in pancreatic α-cells induces pro-convertase-1 expression, shifting glucagon production to GLP-1, hence increasing β-cell mass and function in a paracrine manner [48], while FXR can reduce postprandial glucose utilization by inhibiting hepatic glycolysis and lipogenesis [49]. Interestingly, when researchers focused on the trials performed in developed countries, the beneficial effects of whole grain consumption on glucose metabolism became non-significant. Similarly, a randomized controlled trial conducted in Japan indicated that there is no effect of brown rice intake on glycolipid index, but not for parameters involving endothelial function [32]. On the other hand, researchers also found evidence supporting the beneficial effects of whole grains in subjects with metabolic diseases from a published meta-analysis [50]. Given that the study conducted in Japan did not assess the real increases in fibre intake in an experimental group; the number of local economic level-based studies in the present subgroup analysis is also relatively small. These biases may confound the results and need to be verified with well-designed trials in the future.
Insulin, which acts as a unique hormone that lowers the blood glucose in the body, plays a key role in maintaining the nutrient homeostasis during the postprandial state. It has been reported that insulin can exert many effects in a variety of tissues, including stimulating the influx of glucose into skeletal muscles and promoting the glycogen synthesis; on the other hand, in the liver and adipose tissues, insulin can suppress the production of hepatic glucose, and promote the synthesis and storage of lipids [51]. Indeed, a significant decrease in HOMA-IR value was observed upon whole grain consumption in the study. Similarly, according to Wirstrom et al., whole grain intake showed a negative correlation with HOMA-IR [52]. Moreover, evidence from a high-fat fed-diet animal study suggested that barley β-glucan could improve insulin sensitivity by decreasing serine phosphorylation of insulin receptor substrate 1 and activating Akt and downregulating the mRNA levels of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase [53]. The possible mechanism behind this may be partly attributed to SCFAs, which are produced by bacterial fermentation for dietary fibres. It has been reported that the metabolic actions of SCFAs in improvements of insulin sensitivity including increasing glucose oxidation and insulin clearance and decreasing fatty acid release [54]. Moreover, other metabolites (including lipopolysaccharide, branched-chain amino acid and bile acids) from the gut microbiota may also play an important role in insulin resistance [55]. For example, Faits et al. found that consuming an unrefined carbohydrate diet resulted in a significant increase in abundance of Roseburia, as well as a decreased concentration of secondary bile acid [56]. Results from another randomized controlled crossover feeding study suggested that whole grain consumption led to significant increases in circulating concentrations of taurolithocholic acid, taurocholic acid, and glycocholic acid; moreover, significant associations between bile acids and HOMA-IR were also found in this study [57]. However, the present meta-analysis indicated that whole grain consumption could not improve FPI concentration significantly, even though various subgroup analyses were further conducted. This result is consistent with the meta-analysis performed by Marventano et al., in which they also found that compared with refined foods, the consumption of whole grain is not able to significantly decrease FPI levels in healthy subjects [58]. Of note, when researchers focus on the specific whole grain (e.g., oats), relevant evidence is still needed to be confirmed. Bao et al. suggested that oat intake resulted in a significant reduction in fasting insulin by −6.29 pmol/L [59], while in a meta-analysis performed by Shen et al., there is no significant effect of oat β-glucan intake on FPI, although only two studies were included [60]. Since the diet selections of the control group may vary when studies were performed with different objectives, in current opinion, the types of control diet are very important and should be taken into consideration when exploring the potential biases.
在急性参数方面,目前的结果还显示食用全谷物后 G-iAUC 和 I-iAUC 显着降低。一致地,在健康参与者中进行的荟萃分析也显示,在完全摄入后,G-iAUC 和 I-iAUC 的餐后值显着降低 [ 58 ]。然而,值得注意的是,本研究得出的结果是基于所涉及研究的小样本量,具有较高的异质性。在 Tucker 等人进行的一项随机交叉设计试验中,他们声称全麦酸面包不会显着影响 T2DM 成人的餐后血糖和胰岛素 [ 61]]。研究人员推测,研究之间的差异可能与人群和对照饮食选择的差异有关。此外,由于缺乏相关研究,无法进行亚组分析,对结果的解释要谨慎。
与以往的研究相比,目前的研究具有以下优势:糖尿病相关的代谢参数,包括FPG、胰岛素、HbA1c、HOMA-IR,以及G-iAUC和I-iAUC等急性参数都在考虑范围内。此外,研究人员在探讨异质性的来源时,主要关注食品与国民经济水平相匹配的因素,这些因素可能被其他人忽略,但可能在解释不一致的结果中发挥重要作用。但是,也有一些限制需要考虑。首先,由于缺乏对全谷物的公认定义,控制饮食的选择变得非常重要。不一致的控制饮食可能会导致当前结果出现一些偏差。此外,即使研究人员进行了全面搜索,仍会观察到 FPG 和 HOMA-IR 漏斗图中的显着不对称性。然而,进一步的“修剪和填充”分析为结果的稳健性提供了证据。此外,即使研究人员在亚组分析中将国民经济水平分为发达和不发达,但参与者的真实社会经济地位可能与其国民经济水平不一致。最后,本次荟萃分析纳入的研究数量相对较少,这也可能会影响结果,需要通过更多设计良好的试验进行验证。尽管研究人员在亚组分析中将国民经济水平分为发达和不发达,但参与者的真实社会经济地位可能与其国民经济水平不一致。最后,本次荟萃分析纳入的研究数量相对较少,这也可能会影响结果,需要通过更多设计良好的试验进行验证。尽管研究人员在亚组分析中将国民经济水平分为发达和不发达,但参与者的真实社会经济地位可能与其国民经济水平不一致。最后,本次荟萃分析纳入的研究数量相对较少,这也可能会影响结果,需要通过更多设计良好的试验进行验证。
This entry is adapted from the peer-reviewed paper 10.3390/nu14010109
This entry is offline, you can click here to edit this entry!
