You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
|
Biochemistry & Molecular Biology
BRCA1 has critical functions in accurately repairing double stand breaks in the DNA through a process known as homologous recombination. BRCA1 also has various functions in other cellular processes that safeguard the genome. Thus, mutations or silencing of this tumor suppressor significantly increases the risk of developing breast, ovarian, and other cancers. The objective of this review is to provide significant insights into the mechanisms by which BRCA1 mutations contribute to the metastatic and aggressive nature of the tumor cells.
- BRCA1
- BRCA2
- metastasis
- DNA damage
- DNA repair
1. Introduction
Breast cancer susceptibility gene 1 (BRCA1) encodes the tumor suppressor BRCA1, which was first linked to hereditary breast and ovarian cancer in the early 1990s [1]. Compared to the lifetime risk of developing breast (12.9%) and ovarian cancer (2.7%) in the general population, female carriers of pathogenic BRCA1 mutations are at a significantly higher risk of developing these cancers [2,3]. By age 70, BRCA1 mutations increase the cumulative risk of developing breast cancer to 47–66% and ovarian cancer to 35–46% [4]. BRCA1 mutations frequently give rise to the aggressive, higher-grade, triple-negative breast cancer subtype [5]. Given the critical role of BRCA1 in the repair of DNA double-strand breaks via the error-free homologous recombination (HR) pathway and its additional roles in other cellular processes that safeguard genomic integrity, it is not surprising that mutations in this tumor suppressor gene considerably increase cancer risk [6,7]. Approximately 5% of breast and 15% of ovarian cancer cases were previously thought to arise due to mutations in BRCA1/2; however, HRDetect, a recently proposed weighted model which predicts BRCA1/2 deficiency, has estimated that up to 22% of breast tumors may carry mutations in these genes [8]. HRDetect also identified HR-deficiency in 69% of triple-negative breast cancers, which are more aggressive, associate with poor prognosis, and present a higher risk of recurrence [9]. Therapeutic strategies that benefit cancer patients with BRCA1/2-mutant tumors have shown promising results in targeting tumors with mutations in other genes necessary for HR [10]. Recently, the genome-wide mutational scar-based pan-cancer Classifier of HOmologous Recombination Deficiency (CHORD) estimated that 6% of tumors are HR-deficient with some cancers exhibiting greater prevalence of HR-deficiency (breast: 30%, ovary: 52%) [11]. Thus, treatment strategies that eliminate BRCA1/2-mutant tumors are likely to have a substantial impact on various cancer types and reducing the global cancer burden. Though Poly (ADP-ribose) polymerase (PARP) inhibition is at the forefront of BRCA1/2-mutant breast and ovarian cancer therapy, many new exciting targets such as POLQ, RAD52, FANCD2, FEN1, APEX2, and RNF168, appear to have therapeutic potential in pre-clinical studies [12,13,14,15,16]. These are reviewed in detail elsewhere [17].
While great strides have been made in identifying synthetic lethal interactors of BRCA1, cancer metastasis remains the leading cause of death for all cancers [18]. It is estimated that metastasis is responsible for 60–90% of cancer-related deaths [18,19]. A recent study has also reported that germline BRCA1 mutations in breast cancer patients appear to be associated with an increased risk of brain metastasis even when accounting for other confounding factors, such as age and stage [20].
2. BRCA1 in Cancer Initiation and Metastasis
Mechanisms governing the initiation of BRCA1-associated cancers remain elusive. Given the indispensable function of BRCA1 in maintaining genome stability, it is thought that DNA damage associated with BRCA1 loss results in random mutations in the genome, which inactivate tumor suppressor genes, such as TP53 or activate oncogenes such as MYC, which, through natural selection, promote tumor formation and metastasis [28,39,40,41]. It should be noted that hormonally driven growth during each menstrual cycle produces reactive oxygen species (ROS), which cause oxidative DNA damage leading to DNA lesions, a subset of which produce replication stress requiring repair through HR via BRCA1 [42]. It is also widely believed that long-term and repeated exposure to ROS and estrogen metabolites also contribute to breast cancer risk [43]. In addition, mutations in the tumor suppressor TP53 occur in virtually all BRCA1-mutant cancers and are essential for tumor survival. In a recent study, which examined the spectrum of TP53 mutations in BRCA1/2 associated high-grade serous ovarian cancer identified TP53 mutations in 96% of BRCA1-mutant tumors [44]. This suggests that BRCA1 mutations are not the only determinant of breast tumorigenesis. It is likely that defective HR, compromised genomic integrity, repeated exposure to hormonally driven ROS, and acquisition of additional mutations, all contribute to breast cancer initiation associated with mutations or loss of function of BRCA1.
While metastasis is estimated to account for 60–90% of cancer-related deaths, it remains one of the most poorly understood components of cancer. Metastasis is a complex process comprised of the following steps: (1) local infiltration of cancer cells into adjacent tissue, (2) transendothelial migration of cancer cells into blood vessels (intravasation), (3) survival in the circulatory system, (4) arrest at distant organ sites, (5) extravasation into the parenchyma of distant organs, and finally, (6) survival and proliferation at metastatic sites (Figure 1) [45]. Though little is known about the function of BRCA1 in metastasis, several studies have shown increased frequency of metastasis in carriers of BRCA1 mutations [20,46,47]. Zavitsanos et al. performed a matched pair analysis of breast cancer patients with and without mutations in BRCA1/2 and found that germline BRCA1 mutations in breast cancer patients associated with increased risk of brain metastasis, even when accounting for other confounding factors, such as age, stage, and expression of hormone receptors ER and human epidermal growth factor receptor 2 (HER2) [20]. Song et al. evaluated the patterns of metastasis in breast cancers associated with BRCA1/2 mutations. [48]. Lung and distant lymph node metastases were frequently observed in BRCA1-mutation carriers, whereas bone metastases were frequently observed in BRCA2-mutation carriers [48]. Though this study reported that central nervous system (CNS) metastases were observed at comparable frequencies in both BRCA1- and BRCA2-mutation carriers, when adjusting for breast cancer subtypes, a significant association with CNS metastases was primarily observed in BRCA2 mutation carriers. In another study Ratner et al. assessed whether BRCA1/2 mutations in ovarian cancer increased the risk of brain metastases. This study demonstrated that ovarian cancer patients with mutated BRCA1/2 had a four-fold increased risk of developing brain metastases and were diagnosed with brain metastases approximately 8 months earlier than patients with wild-type BRCA1/2 [46]. Given the increased evidence demonstrating that BRCA1 (and BRCA2) mutations greatly influence the course of BC progression and the risk of metastasis, it is critical to understand the function of this protein in the multistep metastasis process.
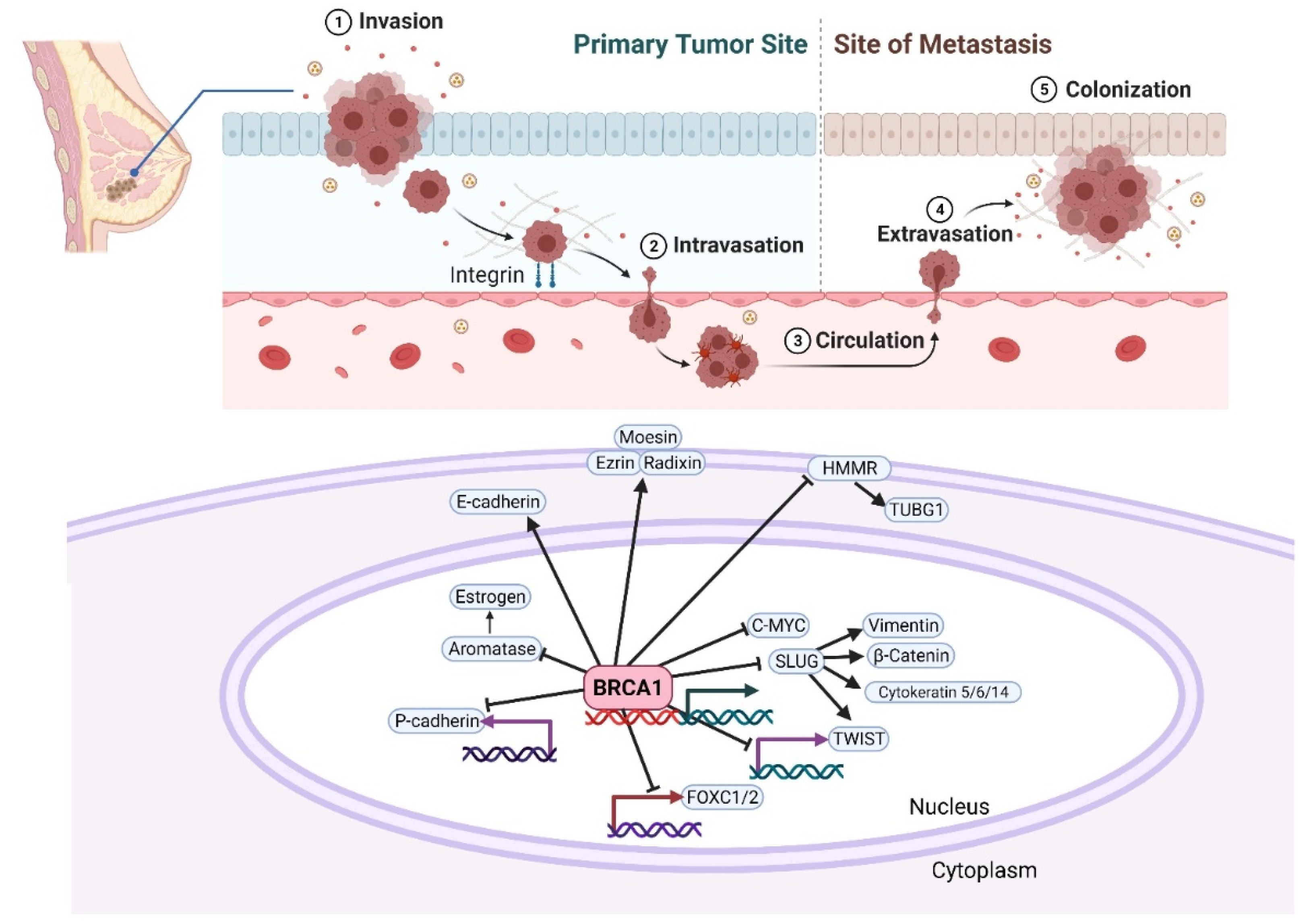
Figure 1. A schematic of the possible effects of BRCA1 on cancer initiation and metastasis. The different stages leading to metastasis are indicated. Several reports have directly or indirectly implicated BRCA1 in the regulation of EMT-promoting factors. BRCA1 mutations in breast cancer cells lead to enhanced EMT phenotype by upregulating key transcription factors like FOXC1/2, TWIST, and SLUG, and by modulating the expression of P-cadherin, cytoskeletal markers, Vimentin, and β-catenin. Breast cancer-associated mutations at the amino-terminus of BRCA1 abolish its ubiquitin ligase activity and abrogate its intracellular colocalization with ERM (Ezrin-Radixin-Moesin) and F-actin, leading to spontaneous motility of human breast cancer cells. Created with Biorender.com.
3. Therapeutic Strategies and Management for BRCA1/2-Associated Metastatic Breast Cancer
Mutations in BRCA1 or BRCA2 genes account for the majority of hereditary breast and ovarian cancers [79]. The penetrance of breast cancer for all BRCA1/2-mutation carriers that have no first-degree relative with breast cancer was 60.4% by age 80 and 63.3% for those with at least one first-degree relative with breast cancer [80]. The clinical management of breast cancer should be the same for both groups of patients, but family history should be taken into account during diagnosis and genetic counseling.
The most effective breast cancer prevention and management for BRCA1/2-mutation carriers is surgical prevention. Although it is invasive and risky, preventive surgery remains an important step in the cancer management of high-risk individuals. Prophylactic mastectomy is one of the most effective ways to prevent breast cancer development in carriers of BRCA1/2 mutations [81]. Patients who had bilateral prophylactic mastectomy had a significantly reduced risk of breast cancer development when compared to BRCA1/2-mutation carriers with two intact breasts. The risk of ovarian, fallopian tube, and peritoneal cancer was reduced by 80% in BRCA1/2-mutation carriers who had undergone preventive bilateral salpingo-oophorectomy (removal of ovaries and fallopian tubes), and was also associated with 77% reduction in all-cause mortality [82,83].
Recent studies have provided sufficient evidence to support the role of chemoprevention agents in high-risk breast cancer patients. Chemoprevention agents include selective estrogen receptor modulators (SERM), such as tamoxifen or aromatase inhibitors (e.g., exemestane) [84]. Tamoxifen is employed as an adjuvant hormonal therapy in ER-positive breast cancer in both pre- and postmenopausal women. Raloxifene, another SERM, is approved only for the treatment of breast cancer in postmenopausal women [85]. Results from two large studies (NSABP-P1 and IBIS-1) showed that tamoxifen treatment reduced the incidence of breast cancer by 40% [86,87]. Interestingly, the treatment was shown to prevent contralateral breast cancer by 50% and demonstrated a 44% risk reduction of developing a second breast cancer in both BRCA1 and BRCA2 WT and mutant conditions [88,89]. Despite significant reduction in breast cancer incidence in pre-menopausal women, the side effects of tamoxifen treatment include increased risk of endometrial cancer and pulmonary embolism in post-menopausal women [90]. The availability of safe and effective drugs may significantly change the rate of high-risk women opting for non-invasive preventive treatments.
Effective development of breast cancer therapeutics requires a full understanding of the mechanisms that drive survival of aggressive breast cancer cells. As BRCA1 and BRCA2 gene products are involved in homologous recombination, recent advances in therapeutic strategies, which increase sensitivity of BRCA1/2-deficient tumors, have provided novel targets for improved treatment of cancers associated with mutations or the loss of expression of these genes [17,91]. BRCA1/2-mutant tumors display exquisite sensitivity to platinum salts such as cisplatin and carboplatin, which act as DNA cross-linking agents [91]. Targeting PARP has emerged as a novel therapeutic strategy utilizing the synthetic lethal interaction between PARP and BRCA1/2 mutations [17,89]. The mechanism of this lethal interaction is associated with the accumulation of DNA double-strand breaks caused by PARP trapping and inhibition [17,92,93]. The treatment of HR defective cancer cells with PARP inhibitors (PARPi) results in persistent DNA double-strand breaks, which lead to cell death [92,93]. A recent report has suggested that PARP inhibitor Olaparib treatment induces changes in the tumor microenvironment of BRCA1-mutated TNBC cells and induces CD8+ T cell infiltration and activation in vivo [94]. It was proposed that the activation of c-GAS-STING pathway results from the cross talk between PARP inhibition and tumor microenvironment [94]. Several PARPi (e.g., Olaparib, Rucaparib, Talazoparib, and Niraparib) have been approved as monotherapy for either breast, ovarian or both cancers associated with BRCA1/2 germline mutations or HR-deficiency [17]. In addition, clinical trials are ongoing to determine the benefit of combination of PARPi with other anti-cancer agents or epigenetic modulators (NCT03901469, NCT04508803).
4. Concluding Remarks and Future Perspectives
BRCA1 is a complex and multifaceted protein implicated in many important biological processes and plays a major role in homologous recombination-mediated DNA double-strand break repair. Although it is well known that BRCA1 functions to maintain genome stability, it is now evident that BRCA1 also plays an important role in cancer cell metastasis by regulating EMT, apicobasal polarity, and the tumor microenvironment. Genes implicated in these processes are likely viable targets to inhibit metastasis in BRCA1 mutation-associated tumors; however, further investigation is required to determine specificity of these processes to BRCA1-deficient tumors considering the commonality in these fundamental processes across tumor subtypes. The various experimental and clinical studies discussed in this review have sought to determine TME changes induced by germline mutation in the BRCA1 gene and how these changes impact tumor behavior and treatment response. In this review, we have attempted to provide meaningful insights into the role BRCA1 plays in controlling cell invasion and metastasis, and the mechanisms by which the changes in TME could lead to breast cancer progression. Future studies should consider the interactions between tumor cells and their microenvironment, with the specific goal of improving cancer therapies for metastatic breast cancer patients.
Aforementioned, BRCA1 mutations or loss-of-function have been linked to different cancers; however, historically, the vast majority of the studies have focused on tissues derived from breast and ovaries. Though this trend continues to this date, in recent years, an interest in examining the role of BRCA1/2 and other DNA repair genes (i.e., ATM, MSH2, etc.) in metastatic prostate cancer and other cancer has emerged. In prostate cancer, BRCA2 mutations are found at a significantly higher frequency of 24.3% compared to BRCA1 mutations (6.4%) [95]. Mechanistically, BRCA1 has been found to interact with the androgen receptor (AR) and functions as a coregulator to enhance AR transactivation in prostate cancer cells [96]. AR plays a pivotal role in prostate cancer [97]. Though androgen deprivation therapy can suppress most prostate cancers, a subset of high-risk tumors can progress to castration-resistant prostate cancers. In fact, AR aberrations are found in 62.7% of metastatic castration-resistant prostate cancers (mCRPC) [98]. An abundance of pre-clinical and clinical evidence implicates AR signaling in the development of both early and late-stage metastatic disease (reviewed in detail elsewhere [99]). BRCA2 has been directly linked to prostate cancer metastasis. Loss of BRCA2 has been demonstrated to promote prostate cancer invasion through up-regulation of matrix metalloproteinase 9 (MMP9) [100]. Indeed, functional BRCA2 protein was found to limit the metastatic potential of cancer cells by downregulating MMP9 production via inhibition of the PI3K/AKT pathway and activation of the MAPK/ERK pathway, thus impairing migration and invasion of prostate cancer cells [100]. BRCA1 (and BRCA2) have indisputable roles in maintaining genomic integrity in various cancers. At present, PARPi (specifically Olaparib and Rucaparib) have been approved for the treatment of mCRPC with germline BRCA1/2 mutations [101]. Several clinical trials are currently underway to examine the efficacy of PARPi alone and in combination with other drugs in BRCA1/2-mutation carriers and in patients with tumors carrying mutations in other DNA repair genes in prostate and other cancers (NCT03148795, NCT04267939). Additional research is necessary to delineate the exact functions of these proteins outside of DNA repair in the development and progression of various cancers.
Herein have summarized recent advances in understanding the functions of BRCA1 in DNA damage repair and breast cancer metastasis. We discussed the implications of BRCA1/2 mutations in the course of breast cancer progression and metastatic recurrence, and also the therapeutic strategies used in the treatment of BRCA1/2-associated metastatic cancers. Understanding the role of BRCA1/2 in tumor development, progression, and metastasis, will help determine the best course of action for patients with mutations in these genes and in patients with HR-defective DNA repair. Identification of metastasis-specific drivers from sequence analyses of biopsies from patients with metastatic tumors might aid in the development of personalized therapy. However, this process can be challenging considering that diversity in mutations may lead to different metastasis. Furthermore, collection of the biopsy samples for these studies poses additional challenges. Advances in the single cell sequencing technologies in combination with high resolution imaging techniques might help understand the processes underlying metastasis and assist in the discovery of druggable vulnerabilities that can suppress metastasis without systemic toxicity.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14010108
This entry is offline, you can click here to edit this entry!
