Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Niacin (also known as “vitamin B3” or “vitamin PP”) includes two vitamers (nicotinic acid and nicotinamide) giving rise to the coenzymatic forms nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP).
- central nervous system
- niacin
1. Introduction
Niacin (also known as “vitamin B3” or “vitamin PP”) is the generic descriptor for two vitamers, nicotinic acid (pyridine-3-carboxylic acid) and nicotinamide (nicotinic acid amide), that give rise to the biologically active coenzymes, nicotinamide adenine dinucleotide (NAD) and its phosphate analog, the nicotinamide adenine dinucleotide phosphate (NADP) [1] (Figure 1). The two coenzymes take part in redox reactions crucial for energy production: in particular, the pyridinic ring can accept and donate a hydride ion (:H−, the equivalent of a proton and two electrons), thus acting as an electron carrier. Nonetheless, NAD and NADP play different metabolic roles in the cytosol: the NADH/NAD+ ratio is small (about 8 × 10−4), thus favoring oxidative catabolism, whereas the NADPH/NADP+ ratio is higher (about 75), thus providing a strongly reducing environment for biosynthetic reactions [2,3].
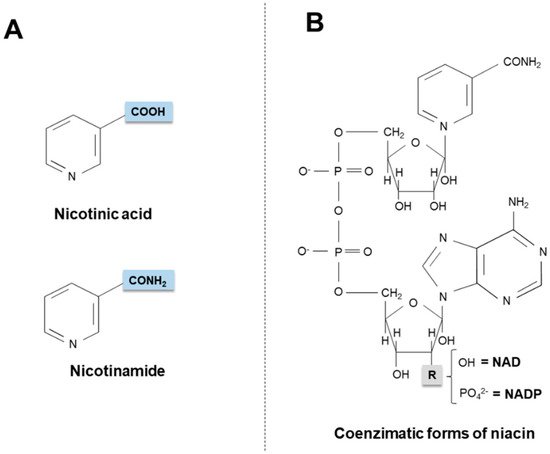
Figure 1. Chemical structures of niacin vitamers (A) and active coenzymatic forms (B). NAD: nicotinamide adenin dinucleotide. NADP: nicotinamide adenin dinucleotide phosphate.
Maintenance of the intracellular NAD pool is not only important to fuel redox metabolism, but also to support NAD-dependent, non-redox signaling pathways. NAD is indeed a substrate of ADP-ribosyltransferases that catalyze ADP-ribose transfer reactions, thus breaking down NAD to nicotinamide and ADP-ribosyl products, which play a key role in cellular signaling cascades regulating gene expression, cell cycle progression, insulin secretion, DNA repair, apoptosis and aging [4,5,6]. Finally, NAD has also been recognized as an endogenous agonist of purinergic P2Y1 and P2Y11 membrane subtype receptors, through which it inhibits neurotransmission in visceral smooth muscles [7] and activates immune cells [8,9], respectively.
2. Niacin Sources
Humans obtain niacin from both endogenous and exogenous sources. Only 2% of dietary tryptophan (Trp) is converted into niacin via a multistep pathway (see in next sections), occurring mainly in the liver [10]. Diet provides the vitamin as nicotinic acid, nicotinamide and Trp, as well as the active coenzymatic forms of niacin.
2.1. Exogenous Sources
Niacin is found in animal and vegetable foods. In meat and fish, the vitamin is present as NAD(P), whose amounts are higher in unprepared foods compared to processed foods (enzymatic hydrolysis of the coenzymes can occur during food preparation).
In mature cereal grains (particularly in corn), niacin is largely present as niacin-glycoside and, in a minor proportion, peptide-bound niacin, compounds collectively termed “niacinogens” [11]. When complexed in niacinogens, niacin is poorly available (only ~ 30%), as intestinal enzymes are not able to free niacin; nonetheless, alkali treatment of the grain increases niacin bioavailability [11].
Once ingested, free niacin can be adsorbed in the stomach, although the small intestine absorbs it faster. The mechanism of transport across the enterocyte brush border membrane is not fully clarified yet. Several transporters, indeed, appear to be involved in intestinal niacin uptake; among them, the most common are the human organic anion transporter-10 (hOAT-10, a proton-driven carrier that also mediates the transport of urate and p-aminohippurate) [12], responsible for niacin uptake at physiological concentrations [13], and the sodium-coupled monocarboxylate transporter (SMCT1 or SLC5A8, a transporter for lactate, pyruvate and short-chain fatty acids), specifically active at high pharmacological doses of nicotinic acid [14,15].
NAD and NADP are quickly hydrolyzed, by intestinal mucosa and liver glycohydrolases, to nicotinamide that is subsequently transported to tissues, where it is converted into coenzymatic forms as necessary. It seems noteworthy that nicotinamide moves freely into or out of the brain [16] and, as discussed in the next sections, such a property has important neurobiological implications.
2.2. Endogenous Synthesis
Starting from dietary Trp, niacin is synthesized via the kynurenine pathway (KP) (Figure 2), occurring mainly in the liver and, to a lesser extent, in extrahepatic tissues (especially upon immune cell activation) [17,18,19].
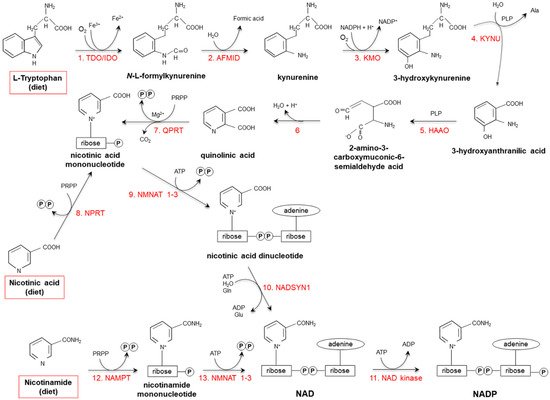
Figure 2. De novo synthesis of NAD(P) from tryptophan, nicotinamide and nicotinic acid. (1) Two iron porphyrin metalloproteins, tryptophan 2,3 dioxygenase (TDO, in the liver) and indolamine-pyrrole 2-3 dioxygenase (IDO, in extrahepatic tissues), oxidize the pyrrole moiety of Tryptophan (Trp), thus forming N-L-formylkynurenine. (2) Arylformamidase (AFMID) hydrolytically removes the formyl group producing kynurenine and is then (3) hydroxylated to 3-hydroxykynurenine by kynurenine-3 monooxygenase (KMO), a mitochondrial flavo-enzyme that uses O2 as a substrate and NADPH as a cofactor. The action of (4) kynureninase B (KYNU, a vitamin B6-dependent enzyme) and (5) 3-hydroxyanthranilic dioxygenase (HAAO, a nonheme iron-dependent dioxygenase) leads to production of 2-amino-3-carboxymuconic-6-semialdehyde acid, an unstable product that (6) spontaneously condensates and rearranges to form quinolinic acid; then, (7) quinolinic acid is decarboxylated and converted to nicotinic acid mononucleotide by quinolinic acid phosphoribosyltransferase (QPRT). Nicotinic acid mononucleotide is also produced through the “salvage pathway”, via the action of (8) nicotinic acid phosphoribosyltransferase (NPRT). The subsequent action of (9) nicotinamide/nicotinic acid-mononucleotide-adenylyltransferases (NMNAT1-3) and (10) NAD synthetase (NADSYN1) leads to the generation of NAD, which is then (11) phosphorylated to produce NADP. NAD can also derive directly from nicotinamide through the action of (12) nicotinamide phosphoribosyltransferase (NAMPT) and (13) nicotinamide/nicotinic acid-mononucleotide-adenylyltransferase (NMNAT1-3). Red frames: dietary precursors of NAD(P). Ala: alanine; Gln: glutamine; Glu: glutamate; PLP pyridoxal phosphate; PRPP: 5-phosphoribosyl-1- pyrophosphate.
Tryptophan 2,3 dioxygenase (TDO), catalyzing the first reaction, is the rate-limiting enzyme. Several nutritional, hormonal and physio-pathological factors affect the efficiency of this anabolic pathway. Deficiencies of vitamin B6, riboflavin, iron and heme (all essential cofactors for specific enzymes), as well as of vitamin B1 and Trp itself, slow the reaction rate [18,20]. Overall: (i) a protein-enriched diet (particularly, consumption of foods with high concentrations of leucine, such as maize or sorghum) decreases niacin biosynthesis; (ii) unsaturated fatty acid-enriched diet increases it, while saturated fatty acids do not exert any effect; (iii) the transformation ratio is higher in diets containing starch with respect to sucrose-rich diets; (iv) caloric restriction drastically suppresses biosynthesis [18,21,22,23,24,25,26].
Among hormones, estrogens, glucorticoids and thyroxine are the best characterized modulators of the KP. Estrogens enhance TDO activity; enzyme activity is triplicated in women who are pregnant or are taking oral contraceptives [27,28]. Glucocorticoids stimulate de novo synthesis, by inducing TDO via a mechanism potentiated by glucagon and inhibited by insulin and adrenaline [18,29,30]. The effects of thyroxine on TDO activity are still controversial, as some studies suggested a positive action, while others did not observe any effect [31,32,33,34].
Due to individual differences, it has been estimated that, in human healthy individuals, Trp is converted to niacin with an average conversion efficiency of 60:1 [35]. Therefore, niacin intakes are expressed as niacin equivalents (NE; 1 mg NE = 1 mg niacin or 60 mg Trp): Recommended Dietary Allowance for adults is 16 mg NE/day for men and 14 mg NE/day for women, with a Tolerable Upper Intake Level of 35 mg/day, based on flushing as the critical adverse effect [36].
3. Pharmacological Effects of Niacin
When supplemented at physiological amounts, nicotinic acid (15–20 mg/day) and nicotinammide (300 mg/day) are effective in treating traditional pellagra [77,78]; nonetheless, at higher concentrations, they display separate additional pharmacological activities, ranging from anti-dyslipidemic to anti-inflammatory action. The first evidence of lipid-altering effects of niacin dates back to 1955, when Altschul and co-workers reported the ability of 3000 mg/day nicotinic acid (but not nicotinamide) to reduce serum cholesterol in humans [79]. An every growing body of experimental data points to beneficial effects of nicotinic acid as an anti-hyperlipidemic agent. It is now well established that nicotinic acid efficaciously: (i) inhibits free fatty acid mobilization and lipolysis; (ii) reduces hepatic triglyceride synthesis and very low density lipoprotein (VLDL) secretion; (iii) inhibits VLDL conversion into low density lipoprotein (LDL); (iv) increases serum high-density-lipoprotein (HDL) levels; (v) triggers LDL conversion from small, dense particles to large, low density particles, (vi) reduces serum lipoprotein concentrations; and (vii) increases apolipoprotein A1 [80,81].
To date, the underlying mechanisms are still speculative; in particular, nicotinic acid (at levels higher than those achieved with diet) has been reported to bind to and activate GPR109A and GPR109B, two G0/Gi-coupled membrane receptors highly expressed in adipose tissue; nonetheless, these receptors are absent, or present only at low levels, in the liver [82]. Therefore, it is conceivable that nicotinic acid might exert its lowering-lipid effects through receptor-independent and -dependent mechanisms.
Due to the above mentioned positive effects, in 2008, nicotinic acid was commercially available as Trevaclyn®, Tredaptive® or Pelzont®, at the dose of 1.0 g (in combination with laropipram, an anti-flushing agent); this prescription product has been used to treat mixed dyslipidemic and/or primary hypercholesterolemic adults receiving statins [83]. However, results from the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial [84], together with the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial [85,86], reported no clinical benefits (i.e., reduced risk of heart attack and stroke) from the long-lasting usage of niacin. A lack of efficacy, together with the onset of recurrent serious side effects (gastrointestinal, musculoskeletal, and skin-related), has led to drug withdrawal from the EU market.
In vitro and in vivo studies have also demonstrated that nicotinic acid (or activation of its molecular targets) exerts significant anti-inflammatory, anti-oxidant and anti-apoptotic activities in a variety of cells and tissues [87], thus being potentially beneficial for the management of several pathological conditions, including type-2 diabetes [88,89], obesity [90,91], atherosclerosis [92], kidney and lung injury [93,94,95], and hyperalgesia [96].
Also nicotinamide at high doses can exert specific pharmacological activities, particularly those related to cancer management. Indeed, several experimental and clinical studies have shown the ability of nicotinamide to sensitize tumors to radiation or chemotherapy [97,98,99,100]. Such an activity depends on activation of poly(ADP-ribose)-dependent apoptosis cascade, as well as on inhibition of myosin light chain kinase that, in turn, enhances microvascular flow, thus improving drug delivery and tumor oxygenation [97,98,99,100].
4. Niacin in the Central Nervous System
Besides dermatitis and diarrhea, niacin/tryptophan deficit symptoms also include several nervous system pathologies, such as dementia and depression, as well as other symptoms resembling those observed in neurodegenerative diseases. This evidence, together with accumulating in vitro and in vivo studies, has underlined the importance of niacin (particularly of nicotinamide) in growth and maintenance of the central nervous system (CNS) [101,102].
Nicotinamide biosynthesis actively occurs in the mammalian brain, which contains nanomolar-low micromolar concentrations of nicotinamide precursors derived from the KP [103,104,105]. Among them, quinolinic acid (unevenly present in different brain regions and, unlike nicotinamide, unable to cross the blood-brain barrier) displays evident neuroactivity [106]: it acts as a N-methyl-d-aspartate (NMDA) receptor agonist, thus causing excitotoxic neuronal lesions and oxidative stress [107]. In addition, quinolinic acid concentrations in the brain (particularly in the cortex) positively correlate with age, thus contributing to neuron synapsis dropout occurring during aging [108]. Finally, neuroinflammation, neurodegeneration and mood disturbs are accompanied by increased quinolinic acid levels in plasma and/or cerebrospinal fluid [10,109,110].
Among KP enzymes, TDO activity is rather low in a healthy human healthy brain [111], where it controls neurogenesis with implications in pre- and post-natal development, as well as in anxiety-related behavior [112]. TDO activity is enhanced under pathological conditions: high activity, indeed, has been found in neurodegenerative diseases and during tumor progression [113,114]. Also indolamine-pyrrole 2-3 dioxygenase (IDO) is expressed in the brain and its activity is increased upon pathological conditions, especially in depression, aging and neuroinflammatory diseases [115,116,117].
Like other vitamins (ascorbic acid, calcitriol and retinoic acid) [118,119,120,121,122], nicotinamide affects neurogenesis by accelerating differentiation of embryonic stem cells or neural progenitors into post-mitotic neurons [123,124]. In vitro vitamin supplementation promotes progression of undifferentiated stem cells to neural progenitors, which further mature into efficient GABAergic neurons; the pro-inducing action is time-dependent as the effects are more pronounced when the vitamin is early received early (day 0) [124]. Accordingly, decreased activity of NNMT (and, therefore, low levels of its metabolic product, N1-methylnicotinamide) is required for regulating pluripotency in stem cells: accumulation of NNMT’s substrates SAM and nicotinamide, indeed, promotes naïve to primed stem cell transition, by making SAM available for histone methylation and regulation of epigenetic events that control the metabolic changes occurring in early human development [125].
Beside the pro-differentiating action, nicotinamide also promotes neuronal survival, especially during oxidative stress conditions, and this effect is achieved via multiple mechanisms, including: (i) prevention of cytochrome c release and caspase 3- and 9-like activities, (ii) inhibition of caspase-3-mediated degradation of forkhead transcription factor (FOXO3a) and (iii) maintenance of protein kinase B (Akt)-dependent phosphorylation of FOXO3a [126].
CNS vascular integrity also positively correlates with NAD levels in brain, where a fine-tuned control of its metabolism occurs. As an example, heterozygous deletion of nicotinamide phosphoribosyltransferase (NAMPT) in the brain exacerbates focal ischemic stroke-induced neuronal death and brain damage [127], while its selective knock down in projection neurons of adult mice leads to motor dysfunction, neurodegeneration and death [128].
Finally, alterations of NAD metabolism are key features of Wallerian degeneration, a process occurring in crushed nerve fibers and leading to degeneration of the axon distal to the injury, representing an early event of age-related neurodegenerative disorders, as well as of chemotherapy-induced peripheral neuropathy [129]. By inducing intra-axonal Ca2+ increase through a pathway requiring the action of the pro-axon death protein SARM1, accumulation of nicotinamide mononucleotide is, indeed, responsible for loss of axonal integrity [130]. The pro-degenerative action of nicotinamide mononucleotide has also been documented during vincristine-induced degeneration in dorsal root ganglion axons [131]. Accordingly, increased activity of nicotinamide/nicotinic acid-mononucleotide-adenylyltransferase (NMNAT) 1–3 protects axons from degeneration, by either limiting nicotinamide mononucleotide levels or activating SIRT1 [132,133].
This entry is adapted from the peer-reviewed paper 10.3390/ijms20040974
This entry is offline, you can click here to edit this entry!
