Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Antioxidants are a class of molecules with an innate affinity to neutralize reactive oxygen species (ROS), which are known to cause oxidative stress. Oxidative stress has been associated with a wide range of diseases mediated by physiological damage to the cells.
- antioxidants
- oxidative stress
- neurotrauma
- neuroregeneration
1. Introduction
Oxidative stress induced by reactive oxygen species (ROS) is inevitably produced from normal cellular metabolism through mitochondrial respiration and a family of membrane-bound NADPH oxidases (NOXs). ROS are broadly divided into free radicals (molecules with unpaired electrons) and non-radical species. Physiologically, three main ROS types are superoxide anion (O2−), hydroxyl radical (OH·), and hydrogen peroxide (H2O2) [1]. ROS play an important role in both physiological cell functioning at low to moderate concentrations by oxidizing different collocated proteins to activate multiple biochemical pathways associated with cell viability, proliferation, differentiation and metabolic adaptation [2]. However, at high concentrations, ROS are toxic and cause oxidation-induced damage to the pivotal cell components including lipids, proteins and DNA leading to cell cycle arrest and cell death [3,4,5,6,7]. There is growing evidence showcasing that the excess oxidative stress cause different pathological diseases including cancer, atherosclerosis, neurological disorders, cardiovascular stress (hypertension, ischemia, reperfusion), diabetes mellitus, chronic inflammation, acute respiratory distress syndrome, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, and asthma [8,9,10]. For example, atherosclerosis is caused by ROS-mediated oxidation of the lipids in low-density lipoprotein (LDL) called lipid peroxidation [11]. In cancer, ROS can promote cancer by introducing conducive genetic mutations by oxidizing specific intracellular chemical moieties and activating biochemical pathways that promote growth and neoplastic transformation [12]. Cancer cells survive high ROS concentrations by preserving the intrinsic concentrations of reduced glutathione and thioredoxin, which allows cells to activate proximal signaling pathways necessary for their neoplastic transformation and simultaneous repair of any damage caused by ROS to inhibit cell death.
Oxidative stress is central to neuropathologies including neurotrauma and neurodegenerative diseases. In neurotrauma, overproduction of ROS, in addition to Ca+2 imbalance, causes continued damage to the central nervous system known as secondary degeneration [13]. This overproduction of ROS causes a deficit in myelin which provides the structural basis for neuronal signal transmission. Damage to myelin causes neuronal damage, which can be exacerbated by the loss of oligodendrocytes (through overexposure to ROS). Oligodendrocytes are more vulnerable to ROS due to their higher intracellular iron levels and lower concentrations of antioxidants (such as glutathione and MnSOD) compared to glial cells [13,14]. ROS was implicated to induce the pathogenesis of neurodegenerative diseases (such as Alzheimer’s and Parkinson’s disease). Although the etiology of neurodegenerative diseases is not entirely understood, however, there is growing support for the role of ROS in augmenting disease progression. It is believed that in AD and PD, commonly found pathologies of aggregation of misfolded proteins can trigger an inflammatory response, which in turn induces ROS production [15,16]. Furthermore, ROS production from mitochondrial dysfunction in neurodegenerative diseases further exacerbates inflammation and dysregulates downstream redox signaling pathways, which were implicated in continued cell death [17]. Given the mixed reports on the efficacy of exogenous therapeutic antioxidants on ROS production in neurodegenerative diseases, there are multiple avenues of investigation currently undergoing to decipher the exact mechanism of ROS on the pathology of neurodegenerative diseases [17,18].
In the normal functioning of cells, ROS generated from mitochondrial functioning gets neutralized by the presence of endogeneous antioxidants. It is to be noted that the amount of endogenous antioxidants is highly cell type-dependent. Quantification of the endogenous antioxidant amount is not trivial despite the availability of assays such as (2,2-diphenyl-1-picrylhydrazyl) DPPH [19,20,21]. Despite this, methods are available to quantify the amount of generated ROS both in vitro and in vivo [22,23,24,25]. Due to the variability in the amount of endogenous antioxidants in different cell types, there has been a drive to explore exogenous antioxidants to supplement the supply and mitigate generating ROS. Exogenous antioxidants can be either natural or synthetic in origin. Natural antioxidants are of two types: enzyme-based and non-enzymatic. Enzymatic antioxidants include catalase [26], glutathione peroxidase [27], and superoxide dismutase [28]. Non-enzymatic antioxidant includes vitamin E [29], ascorbic acid [30], lipoic acid [31], polyphenols [32], and carotenoids [33]. Synthetic antioxidants include curcumin [34], chitosan (CS) [35], carbon nanotubes [36], polyurethane [37], nanoceria [38], melatonin [39], fullerene [40], tannic acid [41], melanin [42], and nitrones [43,44,45,46].
2. Role of Oxidative Stress in Central Nervous System
Groups of free radicals such as superoxide anion, hydroxyl free radical, hydrogen peroxide, hypochlorous acid, singlet oxygen do not come under the exact definition of free radicals and are collectively called ROS [58]. At lower ROS concentrations, they behave as signaling molecules that are involved in cell proliferation and apoptosis and are considered essential for neuronal development and function in the central nervous system (CNS) [59,60]. At higher concentrations, they contribute against pathogens affecting the body. However, when their accumulation increases, they cause cell damage and changes in the DNA causing cell death. ROS are produced as a by-product of oxidation that propagates oxidative chain reactions. When this chain reaction occurs without any known cause and at mild environmental conditions, it is known as autoxidation. When peroxidases are formed as byproducts, it is known as peroxidation. During the oxidative chain reaction, the imbalance between the production and degradation of ROS causes its accumulation leading to cellular damage and cell death [61]. This imbalance is referred to as oxidative stress.
Oxidative stress negatively impacts CNS functions which are mainly because of the damage that occurs to the brain. Increased vulnerability of the brain to oxidative stress is due to its high oxygen demand, presence of high content of polyunsaturated fatty acids (PUFA) in the nerve cell membrane, and accumulation of transition metals [62]. Firstly, the brain is known to use around 20% of the energy of the whole human body due to its high metabolic rate. This energy is produced by mitochondria in the brain cells. Among the brain cells, neurons use about 70–80% of the energy, and the remaining is utilized by the glial cells [63]. Mitochondria produces about 80% of ROS in brain cells which helps in a normal cellular process. Due to leakage of electrons at four complexes (I–IV), ROS levels increase beyond the threshold leading to stress at the molecular level. This increase in ROS level causes DNA oxidation, lipid peroxidation, and protein oxidation. Secondly, the brain also contains a large amount of PUFA majorly docosahexaenoic acid, which increases lipid peroxidation. Lipid peroxidation involves free radical-dependent degradation of lipids. Free radical attack on PUFA present on the neural cell membrane releases highly active aldehydes, which in turn (a) increases the rigidity of the membrane, (b) hinders sodium pump activity, (c) alters membrane permeability, and (d) causes cell damage [64]. Thirdly, the major cause of oxidative stress in CNS is the accumulation of transition metals. Transition metals such as iron, copper, and zinc are required in biological reactions including cell proliferation and differentiation, DNA synthesis, and neurotransmitter synthesis. In addition, iron and copper are known catalysts for ROS production in cells [42]. Due to the increased levels of these metals, ROS production increases, thereby increasing lipid peroxidation leading to oxidative stress and cell damage.
3. Role of Oxidative Stress in Neurodegenerative Diseases
In Parkinson’s disease (PD) genesis, activation of glial cells and enzymes such as reduced nicotinamide adenine dinucleotide phosphate oxidase, inducible nitric oxide synthase, and astrocytic myeloperoxidase, as well as inflammatory factors, such as tumor necrosis factor α and cyclooxygenase-2 play an important role [65]. The progression of PD is mainly attributed to free radical-induced oxidative stress which causes nucleic acid instability, increased mitochondrial DNA mutation, and protein homeostasis disruption [66]. The free radical-mediated oxidative stress damage the dopaminergic neurons in substantia nigra through lipid peroxidation, protein peroxidation, DNA oxidation, alteration in iron content, and monoamine oxidase activation.
In Alzheimer’s disease (AD), oxidative stress biomarkers such as protein carbonyls and 3-nitrotyrosine, 8-hydroxydeoxyguanosine, 8-hydroxyguanosine, 4-hydroxynonenal, and malondialdehyde were shown to increase in the blood. An alteration in the expression of antioxidant enzymes such as superoxide dismutase and catalase was reported to be the cause of familial AD [67]. In AD, β-amyloid is produced in increased quantities causing mutations primarily in the amyloid precursor protein (APP), and presenilin-1 (PS-1), and presenilin-2 genes. β -amyloid level increases with age in human beings [68]. This age-dependent accumulation of β-amyloid was studied by Anantharaman et al. [68] using APP/PS1 transgenic mice. Brain sections were analyzed in the 3-, 6-, 9-, 12-, and 14-month-old mice. There was no deposition of β-amyloid in the 3-month-old mice while 12-month-old mice brains had greater deposition in the neocortex and hippocampus. By 14 months, β-amyloid deposits were seen in small blood vessel walls in the brain and leptomeninges suggesting age-related deposition of β-amyloid in APP/PS-1 mice. It was also observed that the activity of the superoxide dismutase enzyme was significantly decreased when compared to wild-type mice resulting in mitochondrial dysfunction, oxidative stress, and apoptosis. It could therefore be concluded that aging causes an increase in β-amyloid levels leading to the inactivation of superoxide dismutase enzyme and thereby increasing ROS levels which can lead to neuronal death associated with oxidative stress (Figure 1).
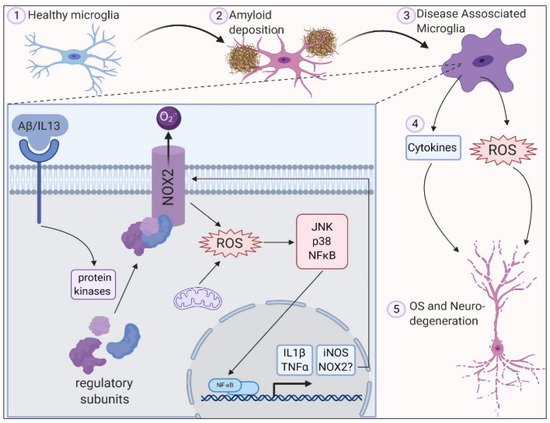
Figure 1. ROS is generated in microglia by activation of NADPH oxidase 2 along with deposition of amyloid plaques and inflammation leading to oxidative stress and neurodegeneration. Reproduced with permission from Simpson et al. [10].
In Amyotrophic Lateral Sclerosis (ALS), both upper and lower motor neuron loss is seen which results in muscle wasting, weakness, and paralysis. Its etiology is unknown but superoxide dismutase enzyme mutations are observed [69]. Mutation in the superoxide dismutase-1 enzyme forms protein aggregates which cause loss of enzymatic function. Superoxide dismutase-1 enzyme plays an important role in the clearance of free oxygen radicals. With the loss of enzymatic function in ALS, the accumulation of oxygen radicals increases leading to oxidative stress.
This entry is adapted from the peer-reviewed paper 10.3390/antiox11010072
This entry is offline, you can click here to edit this entry!
