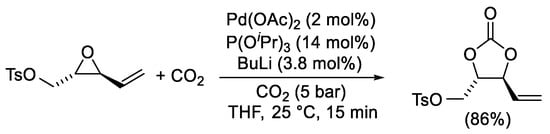The efficient incorporation of carbon dioxide into an organic substrate (carboxylation) under catalytic conditions to give high value added molecules is one of the most important and fascinating areas of current organic synthesis. Carbon dioxide is a nonflammable, inexpensive and largely available C-1 feedstock. In fact, it allows converting an important waste (it is well known that carbon dioxide is produced in enormous amounts from the combustion of fossil fuels for the production of energy) into a variety of useful compounds, which can find application as fuels or in the pharmaceutical or material fields.
1. Palladium-Catalyzed Incorporation of Carbon Dioxide into Olefinic Substrates
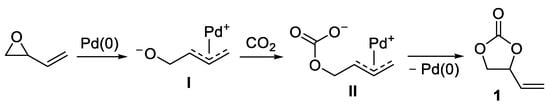
Suitably functionalized olefins are excellent substrates for the Pd-catalyzed incorporation of carbon dioxide to give high value added heterocyclic compounds. For example, vinyl epoxides undergo a Pd(0)-promoted ring-opening process with formation of a π-allylpalladium alkoxide intermediate
I, which can attack CO
2 to give a π-allylpalladium carbonate
II that then undergoes intramolecular nucleophilic attack to give vinyl-substituted 5-membered cyclic carbonates
1, as shown in
Scheme 1. This kind of process was independently disclosed by the groups of Fujinami
[1] and Trost
[2][3] in the 1980s; an example of synthetic application is shown in
Scheme 2 [4].
Scheme 1. Pd(0)-catalyzed carboxylation of 2-vinyloxirane to 4-vinyl-1,3-dioxolan-2-one 1.
Scheme 2. Synthesis of trans-(2-oxo-5-vinyl-1,3-dioxolan-4-yl)methyl 4-methylbenzenesulfonate.
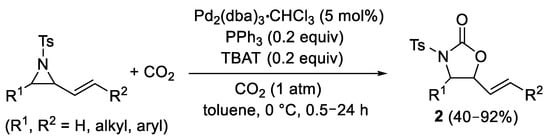
In a similar manner, 5-vinyloxazolidinones
2 can be synthesized from vinylaziridines. For example, using a catalytic system consisting of Pd
2(dba)
3/PPh
3/TBAT (TBAT = tetrabutylammonium difluorotriphenylsilicate), in toluene as the solvent and under particularly mild reaction conditions (0 °C and atmospheric pressure of CO
2), a variety of 5-vinyloxazolidinones was prepared (
Scheme 3). The process showed high regioselectivity and was also diastereospecific
[5].
Scheme 3. Synthesis of 5-vinlyloxazolidinones 2 from vinylaziridines.
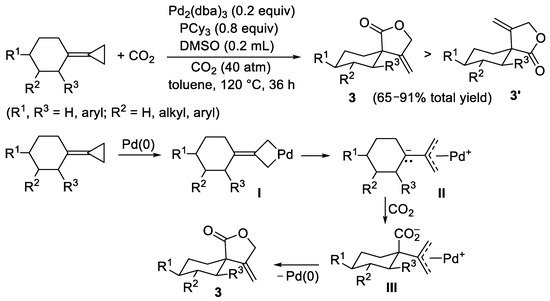
Cycloalkylidenecyclopropanes, bearing the highly reactive cyclopropane ring, have been reported to undergo ring-opening carboxylation under relatively mild conditions to yield five-membered lactones, as shown in
Scheme 4 for the formation of
3 and
3′ [6]. Reactions were carried out in toluene at 120 °C under 40 atm of CO
2, in the presence of Pd
2(dba)
3/PCy
3 as the catalytic system and dimethyl sulfoxide (DMSO) as additive. The reaction showed a certain degree of diastereoselectivity, favoring the formation of the diastereoisomer with the carbonyl group in the axial position
3 in most cases. The proposed mechanism involves the initial oxidative insertion of Pd(0) into the cyclopropane ring with formation of a four-membered Pd(II) palladacycle intermediate
I, as shown in
Scheme 4, which may undergo a ring opening process to give a zwitterionic π-allypalladium complex
II. The latter inserts CO
2 by carbanion attack to give a carboxylate zwitterionic intermediate
III (only the intermediate leading to the major diastereoisomer is shown in the scheme), from which the final product
3 is formed by intramolecular attack of the carboxylate group to the π-allyl moiety, with regeneration of the Pd(0) catalyst (
Scheme 4).
Scheme 4. Synthesis of five-membered lactones 3 and 3′ from cycloalkylidenecyclopropanes.
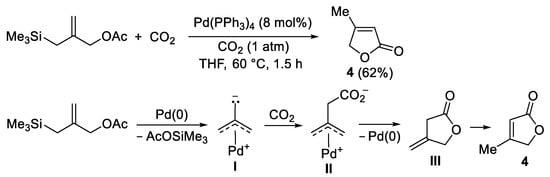
Functionalized allylic substrates can also undergo carbon dioxide fixation under Pd(0) catalysis. As an example, 2-(acetoxymethyl)-3-(trimethylsilyl)propenes were converted into 2(5
H)-furanones when allowed to react with CO
2 (1 atm) in 1,2-dimethoxyethane (DME) or THF at 60–75 °C in the presence of Pd(PPh
3)
4, although in modest to moderate isolated yields (35–62%), as exemplified in
Scheme 5 for the formation of
4 [7]. Mechanistically, the reaction follows a pathway similar to that seen in
Scheme 1, as the key intermediate is a zwitterionic π-allylpalladium complex
I [formed by oxidative addition of the substrate to Pd(0)], which reacts with CO
2 to give a zwitterionic carboxylate complex
II. The latter undergoes cyclization (by intramolecular nucleophilic attack of the carboxylate to the π-allylpalladium system), with regeneration of Pd(0) and formation of a 4-methylenedihydrofuran-2(3
H)-one intermediate
III, which eventually isomerizes to the final 2(5
H)-furanone product
4 (
Scheme 5).
Scheme 5. Synthesis of 4-methylfuran-2(5H)-one 4 from 2-((trimethylsilyl)methyl)allyl acetate.
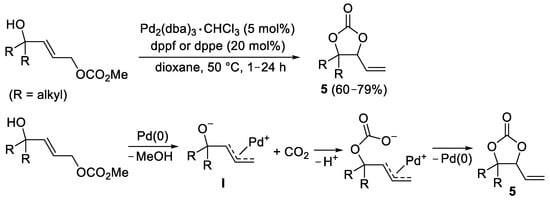
Vinyl-substituted 5-membered cyclic carbonates
5 were synthesized from allylic carbonates by a sequential CO
2 elimination–fixation process, as shown in
Scheme 6, with formal CO
2 recycling, again through the formation of a zwitterionic π-allylpalladium complex
I [8]. Reactions were performed in the presence of Pd
2(dba)
3 as catalyst in the presence of dppf [1,1′-bis(diphenylphosphino)ferrocene] or dppe [1,2-bis(diphenylphosphino)ethane] as ligand, in dioxane at 50 °C under inert atmosphere. The process was shown to be enantiospecific when starting from nonracemic allylic carbonates to yield nonracemic cyclic carbonates.
Scheme 6. Synthesis of 4-vinyl-1,3-dioxolan-2-ones 5 from (E)-4-hydroxybut-2-en-1-yl methyl carbonates.
2. Palladium-Catalyzed Incorporation of Carbon Dioxide into Acetylenic Substrates
Acetylenic substrates have been reported to undergo several important carboxylation processes catalyzed by palladium, with formation of high value added compounds.
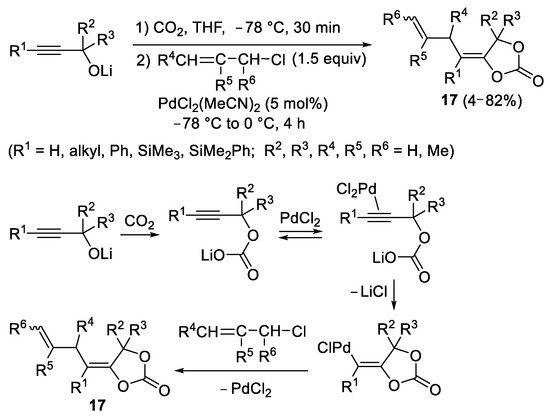
In 1986, Utimoto and coworkers reported the Pd(II)-catalyzed reaction of lithium 2-alkynyl carbonates (obtained from the reaction between lithium 2-alkyn-1-olates with CO
2) with allylic chlorides to give 4-(but-3-en-1-ylidene)-1,3-dioxolan-2-ones
17 (
Scheme 7)
[9]. The synthetic transformation was carried out in a one-pot, two-step fashion, by allowing ynolate to react with CO
2 first (in THF at −78 °C) and then adding the allyl chloride together with the catalyst PdCl
2(MeCN)
2 at the same temperature followed by stirring at 0 °C for 4 h, as shown in
Scheme 7.
Scheme 7. Synthesis of 4-(but-3-en-1-ylidene)-1,3-dioxolan-2-ones 17 from lithium 2-alkyn-1-olates.
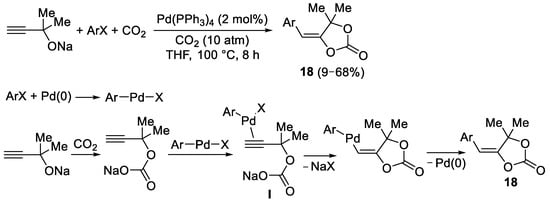
Later on, Inoue and coworkers reported the Pd(0)-catalyzed reaction between sodium 2-alkyn-1-olates bearing a terminal triple bond, aryl halides, and CO
2 to give cyclic carbonates
18 [(
E)-4-(arylmethylene)-1,3-dioxolan-2-ones], as shown in
Scheme 8 [10]. Reactions were carried out under 10 atm of CO
2, in THF at 100 °C and in the presence of 2 mol% of Pd(PPh
3)
4. In this process, the initial oxidative addition of the aryl halide to Pd(0) leads to an Ar–Pd–X complex, which electrophilically activates the triple bond toward the
anti 5-
exo-
dig intramolecular nucleophilic attack by the carbonate anion
I formed by the reaction between the ynolate and CO
2 (
Scheme 8).
Scheme 8. Synthesis of (E)-4-(arylmethylene)-1,3-dioxolan-2-ones 18 from sodium 2-alkyn-1-olates and aryl halides.
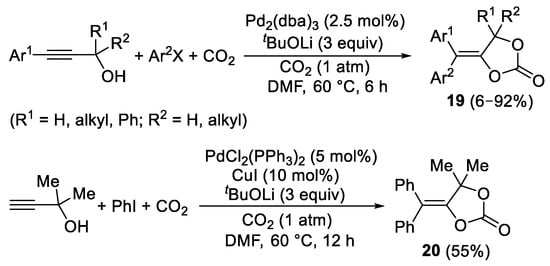
2-Ynolates can also be formed in situ starting by deprotonation of propargyl alcohols in the presence of a suitable base. For example, recently a variety of propargyl alcohols with the triple bond substituted with an aryl group were converted into 5-(diarylmethylene)-1,3-dioxolan-2-ones
19 by their Pd(0)-catalyzed reaction with aryl halides and CO
2 (1 atm), carried out in the presence of Pd
2(dba)
3 as catalyst and
tBuOLi as the base (
Scheme 9)
[11]. Interestingly, with substrates bearing a terminal triple bond, a sequential Sonogashira coupling–carboxylation process took place, as exemplified in
Scheme 9 for the case of the reaction of 2-methylbut-3-yn-2-ol with PhI (3 equiv) and CO
2. The reaction, carried out in the presence of 5 mol% of PdCl
2(PPh
3)
2 as the catalyst precursor, CuI (10 mol%) as cocatalyst, and
tBuOLi as base (3 equiv), led to formation of 5-(diphenylmethylene)-4,4-dimethyl-1,3-dioxolan-2-one
20 in 55% yield (
Scheme 9).
Scheme 9. Synthesis of 5-(diarylmethylene)-1,3-dioxolan-2-ones 19 and 20 from propargyl alcohols and aryl halides.
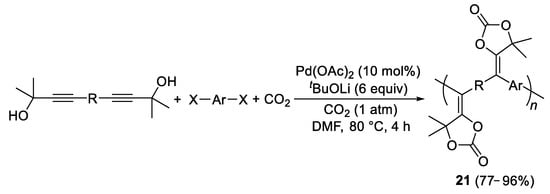
Polymeric materials can be obtained starting from bis(propargylic alcohol)s and aryl dihalides. Thus, linear and hyperbranched five-membered cyclic carbonate-based polymers
21 with high weight-average molecular weights (up to 42,500, 96% yield) were recently produced by allowing to react bis(propargylic alcohol) monomers and aryl dihalide monomers with CO
2 (1 atm) in DMF in the presence of Pd(OAc)
2 as catalyst precursor and
tBuOLi as the base (
Scheme 10)
[12].
Scheme 10. Formation of five-membered cyclic carbonate-based polymers 21 from bis(propargylic alcohol)s and aryl dihalides.
3. Palladium-Catalyzed Incorporation of Carbon Dioxide into Other Substrates
Under suitable conditions, aryl halides and triflates can undergo palladium-catalyzed carboxylation with formation of important compounds.
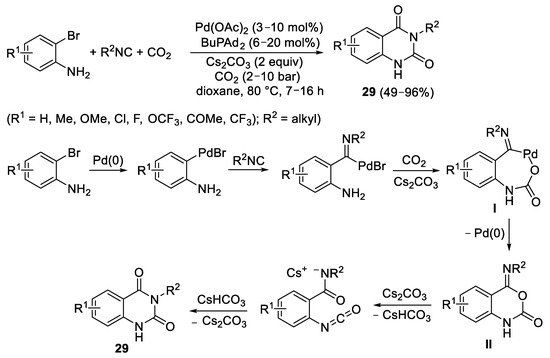
In 2017, the groups of Maes and Beller reported the Pd(0)-catalyzed reaction of 2-bromoanilines with CO
2 and isocyanides to give quinazoline-1,4(1
H,3
H)-diones
29 [13]. Reactions were performed in the presence of Pd(OAc)
2 as the catalyst precursor, in the presence of BuPdAd
2 (Ad = adamantly) as ligand and Cs
2CO
3 as base, in dioxane as the solvent at 80 °C and under 10 bar of CO
2 (
Scheme 11). In the simplified version of the mechanism, oxidative addition of the Ar–Br bond to Pd(0) takes place, followed by insertion of the isocyanide. The reaction of the amino group with CO
2 in the presence of the base then leads to a palladium carbamate intermediate
I, which undergoes reductive elimination to yield Pd(0) and a 4-imino-1,4-dihydro-2
H-benzo[
d][1,3]oxazin-2-one intermediate
II, from which the final product is obtained by base-promoted rearrangement (
Scheme 11).
Scheme 11. Synthesis of quinazoline-1,4(1H,3H)-diones 29 from 2-bromoanilines and isocyanides.
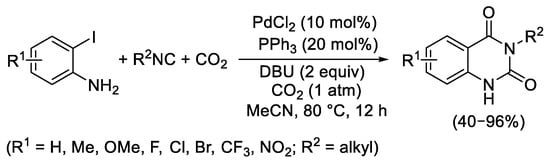
In the same year (2017), independently, the group of Wang and Ji published exactly the same transformation from 2-iodoanilines under atmospheric pressure of carbon dioxide, using PdCl
2 in the presence of PPh
3 as the catalyst precursor and DBU as the base, in MeCN at 80 °C (
Scheme 12)
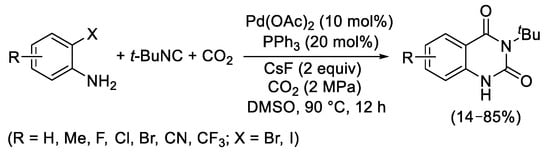
[14]. Additionally, in 2018 Zhang and coworkers reported the use of both 2-bromo- and 2-iodoanilines using catalytic amounts of Pd(OAc)
2 in the presence of PPh
3 as ligand and CsF as base, under 2 MPa of CO
2, in DMSO at 90 °C (
Scheme 13)
[15].
Scheme 12. Synthesis of quinazoline-1,4(1H,3H)-diones from 2-iodoanilines and isocyanides.
Scheme 13. Synthesis of quinazoline-1,4(1H,3H)-diones from 2-haloanilines and tert-butyl isocyanide.
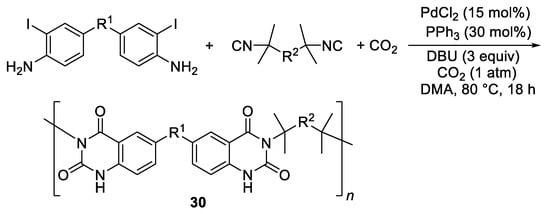
Interestingly, very recently this kind of reactivity has been exploited for the synthesis of new heterocyclic polymers
30 with self-assembly and sensing properties, starting from bis(2-iodoaniline) and diisocyanide monomers, using PdCl
2 and PPh
3 as the catalyst precursor, under 1 atm of CO
2, in DMA at 80 °C for 18 h (
Scheme 14)
[16].
Scheme 14. Synthesis of heterocyclic polymers 30 from bis(2-iodoaniline)s and diisocyanides.
4. Conclusions
The versatility of palladium-based catalysts has been successfully exploited also in the efficient conversion of carbon dioxide into high value added organic molecules of applicative and pharmacological interest. Particularly important results have been achieved in the Pd(0)-promoted CO2 incorporation into small rings (such as suitably functionalized epoxides and aziridines) as well as into suitably functionalized alkenes, allenes, or alkynes, to give highly important heterocyclic derivatives, such as cyclic carbonates, oxazolidinones, etc. Under Pd(II) catalysis, particularly important results have been achieved with propargyl amines as substrates, with formation of oxazolidinones, which could also incorporate an exocyclic estereal function working in the presence of CO together with CO2 under appropriate conditions.
On this grounds, it is expected that in the future palladium will play a major role in CO2 utilization and incorporation into suitable substrates, possibly in the presence of other promoting species (either metal-based or organo-based) as cocatalyst(s), which will make it possible to achieve more demanding processes for the direct and selective synthesis of complex molecular architectures.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27010262