Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Necrotizing enterocolitis (NEC) is a significant cause of mortality and morbidity in preterm infants. The pathogenesis of NEC is not completely understood; however, intestinal immaturity and excessive immunoreactivity of intestinal mucosa to intraluminal microbes and nutrients appear to have critical roles.
- fatty acids
- necrotizing enterocolitis
- intestinal inflammation
1. Introduction
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in preterm infants and is a major cause of mortality and morbidity in the neonatal intensive care unit (NICU). A systematic review and meta-analysis indicated that confirmed NEC is associated with 23% mortality, 24–61% neurodevelopmental disability, and 15–35% intestinal failure [1]. The pathogenesis of NEC is multifactorial and not completely understood. Intestinal immaturity, abnormal microbial colonization, ischemic-reperfusion injury, and highly immunoreactive intestinal mucosa are all thought to be important factors that may lead to NEC [2]. NEC occurs mostly in infants on enteral feeds. Feeding human milk, either the mother’s own milk or donor human milk, remains the most protective measure against NEC. Several studies including randomized trials have shown that the incidence of NEC is 2- to 10-fold lower in human milk-fed infants compared with formula-fed infants [3]. Formula-fed infants are typically at the highest risk for NEC; therefore, several formula components including fat are suggested to play a critical role in the disease development [4]. In contrast, feeding breastmilk has often been shown not only to prevent NEC, but also to promote gastrointestinal health [5].
Preterm infants have higher energy needs than their term counterparts in the early postnatal life [6][7]. Fat provides approximately 50% of the preterm infant’s caloric needs and supports many physiologic and metabolic functions that are vital to their growth and neurodevelopment [8][9][10]. Furthermore, several fatty acids play fundamental immunomodulatory roles by regulating key pathways for inflammatory responses [9][11]. Human milk contains long chain polyunsaturated fatty acids (LC-PUFAs), mainly arachidonic acid (AA) and docosahexaenoic acid (DHA), that modulate intracellular signaling within immune cells. A balance between the n6 LC-PUFAs and the n3 LC-PUFAs promotes lipid mediator formation that is crucial to achieving protection against pathogens without exaggerated inflammation [12].
2. Postnatal Fatty Acid Status in Preterm Infants
In the third trimester of pregnancy, the fetus increases its nutrient demands to support rapid tissue growth with fat deposition. Preterm birth interrupts the placental transfer of the fatty acids. Parenteral lipid has become the standard of care to meet the early fat requirements of preterm infants after birth. Following the weaning of parenteral lipid, preterm infants rely on enteral sources to meet these requirements, whether through breastmilk and or formula. Since the fat content of the intravenous lipid emulsions (IVLEs) and enteral nutrition is inadequate to meet preterm infants’ needs for more than a few days, postnatal deficits of certain fatty acids accumulate quickly. For example, the DHA status of extremely low birth weight (ELBW) infants declines significantly in the first 2 months after birth, particularly in infants exposed to IVLEs for more than 28 days [13]. This low DHA status can remain for weeks, even after the establishment of full enteral feeding [14]. Several other plasma fatty acids abnormalities such as low (AA) and high linoleic acid (LA) are also described in very preterm infants [15]. Some of these abnormalities are associated with neonatal morbidities. A study by Martin et al. revealed that the deficit in DHA was associated with increased risk of bronchopulmonary dysplasia (BPD), while the decreased AA and high LA levels were associated with increased risk of nosocomial sepsis [15]. The number of infants who developed NEC in the former study was limited to 5, making it difficult to examine the association between plasma fatty acids levels and NEC [15]. Nevertheless, common morbidities in preterm infants often involve elements of uncontrolled inflammation. Current evidence suggests that alterations in LC-PUFA delivery to preterm infants may have negative implications on the risk of neonatal morbidities including NEC [11][16][17].
3. Fatty Acids of Breastmilk
Feeding human milk has been shown to improve gastrointestinal function and reduce the incidence of NEC. Term and preterm infants are born with an immature immune response. The maturation of an infant’s immune system is supported by breast milk fatty acids including the LC-PUFAs AA and DHA. Unlike infant formulas, the fatty acid profile of human milk is influenced by maternal diet. Despite maternal diet variability, breastmilk provides a relatively constant n6 to n3n3 ratio. LCPUFAs may contribute to the immune benefits of human breast milk; however, the extent of this contribution is unclear [12].
3.1. Fatty Acids Content and Structure
The average human milk fat content is 3.5 g/100 mL with wide variation between 1.8 and 4.9 g/100 mL [6]. This variation depends on many factors such as lactation period, feeding stages (foremilk and hindmilk), and dietary habits of mothers [18][19]. For donor human milk, it is further influenced by processing and pasteurization [20].
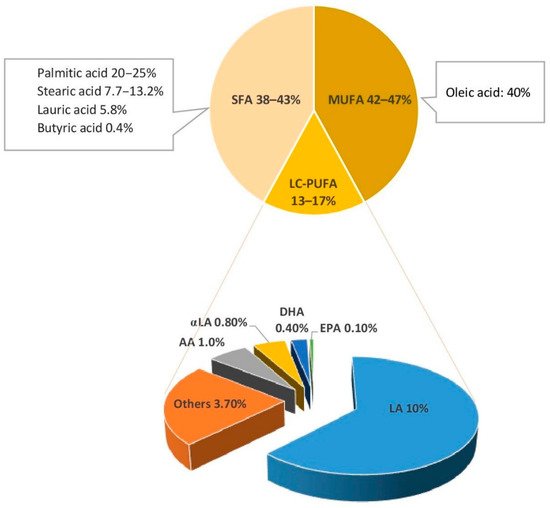
Triglycerides (TGs), which compose 98% of breastmilk fat, consist of two main components: a glycerol backbone and three fatty acids tails [21][22]. The structure of these fatty acids depends on a number of molecular characteristics including the number of carbon atoms, the presence or absence of unsaturated bonds, their number, and their position in the TG molecule [21]. Fatty acids are classified as saturated or unsaturated according to the number of double bonds in the hydrocarbon chain. Monounsaturated fatty acids (MUFAs) have one double bond, whereas polyunsaturated fatty acids (PUFAs) have several. LC-PUFAs (18 or more carbons) comprise 90% of all PUFAs in breastmilk [14]. They are classified into two key families: n3 and n6, depending on the position of the first double bond in the hydrocarbon chain. Breastmilk typically contains 35–40% saturated fatty acids (SFAs), 45–50% MUFAs, and approximately 15% LC-PUFA [23]. Figure 1 shows the type and concentration of fatty acids of breastmilk.
3.2. Role of Fatty Acid Balance in the Infant Diet
LC-PUFAs have many structural, energetic, and metabolic functions. LA (n6) and α-linolenic acid (αLA; n3) are PUFAs known as ‘essential fatty acids’ (EFAs). EFAs are converted into metabolites to exert pro- and anti-inflammatory actions. LA is converted to AA (n6), which is the main precursor of thromboxanes, prostaglandins, and leukotrienes. These metabolites are common precursors for the inflammatory response of NEC [25]. Derivatives of αLA include DHA (n3) and eicosapentaenoic acid (EPA; n3). DHA and EPA are precursors of series-3 prostaglandins that inhibit platelet aggregation [26].
Preserving an appropriate ratio between SFAs, MUFAs and LC-PUFAs, and between the n3 and n6 LC-PUFAs derivatives, DHA and AA, is important to maintain intestinal cytokine balance and prevent intestinal necrosis and apoptosis. Animal studies showed that modifying fatty acid composition of the early diet influences intestinal membrane fatty acids, with effects on permeability and inflammatory pathways that persist into later life [27][28]. Suppression of AA (n6; LC-PUFAs) by feeding female rats diets with 20% energy from safflower oil (15% oleic acid; n9 MUFA and 75% LA; n6 LC-PUFA) or canola oil (60% oleic acid; n9 MUFAs and 21% LA; n6 PUFA), 8% fish oil (n3 PUFA) plus 2% soy oil (51% LA and 23% oleic acid), or 18% fish plus 2% soy oil throughout gestation and lactation resulted in significant aberration in the normal trajectory of intestinal development with reduced crypt depth in their pups on a fish oil diet [28]. Moreover, the intestine of pups on the fish oil diets had a long-lasting heightened inflammatory response to experimental colitis in their young adulthood [28]. Another study by Van Greevenbroek et al. showed that supplementation with palmitic acid (SFA) resulted in disturbances in cell morphology and intracellular accumulation of TG precursor molecules in intestinal Caco-2 cells compared to supplementation with oleic acid (MUFAs) and LA (n6 PUFAs) [29][30].
A diet high in SFAs outside of the context of a breastmilk-based diet has been shown to increase metabolic stress and risk of adverse health outcomes [31][32]. Most preterm infant formulas are manufactured to match the fatty acids content of breastmilk and achieve similar balance between SFAs, MUFAs, and LC-PUFAs. However, the absence of breastmilk fat digestive enzymes and the low pancreatic lipase activity in preterm infants are not usually considered when comparing the differences in bioactivity, functions, and intestinal absorption capacity between formula and breastmilk. Recently, a pre-clinical study suggested potential benefits of using predigested fat in formula. [33].
The use of predigested macronutrients to improve the intestinal health of preterm infants is not new. Protein hydrolysate formula has been frequently proposed to improve protein absorption and promote feed tolerance. To date, there is no strong evidence to support the notion that a hydrolyzed protein diet lowers the incidence of NEC or feeding intolerance as it relates to the digestibility and absorption of proteins [34]. There are also no studies that have examined the effect of predigested fat on the incidence or severity of NEC in human preterm infants. In both cases, the lack of evidence to support the benefits of predigested macronutrients warrants further research in this area.
4. Fatty Acids in Infant Formula
There are many term formulas and nutrient-enriched preterm formulas available to neonates. Term formula is manufactured to mimic mature human breast milk. The amount of protein, calcium, and phosphate in term formula does not meet the estimated nutrient requirements of preterm infants. In contrast, preterm formula is energy and protein-enriched with higher amounts of vitamins, minerals, and trace elements than term formulas. Preterm formulas are designed to meet intrauterine nutrient accretion rates. The fat content of these formulas is also meant to model that of human milk [35].
Additions of LC-PUFAs to preterm and term formulas have been largely influenced by early reports on positive effects on cognitive development and visual acuity [36][37]. The design of previous studies and the content of current infant formulas may be influenced by these reported benefits. This may have contributed to the lack of strong evidence in NEC.
4.1. Fatty Acids Content in Preterm Infant Formulas and Human Milk Fortifiers
As in human milk, the dominant lipids in term and preterm infant formula are TGs. The lipid compositions of infant formulas and human milk fortifiers (HMFs) vary according to the fat sources used to manufacture them. The fat content of most infant formulas is formulated to match the concentration of fat in human milk. Nevertheless, some preterm formulas have 36–43% less fat than donor human milk [38]. HMFs are also designed to match the fat content of breastmilk; however, current evidence suggests that both standard and targeted milk fortification methods lead to fat content that is higher than the recommended intake [39]. It is worth noting that reaching fat content higher than the recommended intake is less likely when using fortified donor human milk.
4.2. Source of Fatty Acids in Infant Formula
Vegetable oils are commonly used to provide fatty acids in preterm and term infant formulas and HMFs. The fatty acids found in vegetable oils do not provide adequate amounts of LC-PUFA derivatives, EPA, DHA, and AA [38][40][41]. As a result, most infant formula manufacturers add AA and DHA to their products using fish, algal, or fungal oils [42]. Preterm and term infant formulas contain a high concentration of MUFAs. Oleic acid content has been reported to be higher in some preterm infant formulas than breastmilk [38][43][44].
4.3. n3 and n6 LC-PUFAs Balance in Infant Formula
In general, most infant formulas have a wide range of n3 and n6 LC-PUFAs. Given that n3 and n6 PUFAs are competitively metabolized by the same set of desaturation, elongation, and oxygenase enzymes, it is crucial to maintain their balance in infant formula. The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) recommends the ratio of LA to αLA to be 5:1 to 15:1 [42]. The proposed amount of LA in infant formula is 0.3–1.2 g/100 kcal [42]. When DHA is added to infant formula, it should represent 0.5–1.0% of the total fat content [45].
The addition of LC-PUFAs to preterm formula has theoretical and clinical benefit [46]. LC-PUFAs, particularly the balance between AA and DHA, have important clinical and immunomodulatory roles during the postnatal period when the immune system is rapidly developing. A recent cohort study found that higher mean daily serum levels of DHA during the first 28 postnatal days is associated with less severe retinopathy of prematurity (ROP) even after adjustment for known risk factors. This effect was only seen in preterm infants with sufficiently high AA levels [47]. In this study, the incidence of NEC was three times higher in the infants with severe ROP compared to those with no or mild to moderate ROP [47]. For term infants, formulas enriched in both LC-PUFAs in ratios similar to that of breastmilk have shown to alter immune function markers more comparable to those of exclusively breastfed infants [21][23]. In contrast, feeding infants a diet with high doses of n3 LC-PUFA without additional AA, reduces the n6:n3 LC-PUFA ratio and results in immunosuppressive and anti-inflammatory effects through the reduction in the cell content of AA. [21]. This unbalanced diet is undesirable in the early postnatal period, particularly in preterm infants when the immune system is rapidly developing. Similar findings of the benefits of an optimal ratio between DHA and AA are observed in animal models undergoing experimental NEC. Caplan et al. revealed that the combination of AA and DHA in neonatal rats attenuates the degree of experimental NEC by reducing intestinal inflammation [48]. DHA alone was unable to show any beneficial effect in terms of reducing NEC or Toll-Like Receptor (TLR) 4 expression [48].
This entry is adapted from the peer-reviewed paper 10.3390/nu14010145
References
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3.
- Neu, J.; Walker, W.A. Medical Progress: Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264.
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1322.
- Sinclair, T.J.; Ye, C.; Chen, Y.; Zhang, D.; Li, T.; Ling, X.B.; Cohen, H.J.; Shaw, G.M.; Stevenson, D.K.; Chace, D.; et al. Progressive Metabolic Dysfunction and Nutritional Variability Precedes Necrotizing Enterocolitis. Nutrients 2020, 12, 1275.
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080.
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216.
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59.
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279.
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17.
- Kim, Y.; Himmelsbach, D.S.; Kays, S.E. ATR-Fourier Transform Mid-Infrared Spectroscopy for Determination of trans Fatty Acids in Ground Cereal Products without Oil Extraction. J. Agric. Food Chem. 2007, 55, 4327–4333.
- Lapillonne, A.; Moltu, S.J. Long-Chain Polyunsaturated Fatty Acids and Clinical Outcomes of Preterm Infants. Ann. Nutr. Metab. 2016, 69, 35–44.
- Miles, E.; Childs, C.; Calder, P. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247.
- Robinson, D.T.; Carlson, S.E.; Murthy, K.; Frost, B.; Li, S.; Caplan, M. Docosahexaenoic and Arachidonic Acid Levels in Extremely Low Birth Weight Infants with Prolonged Exposure to Intravenous Lipids. J. Pediatr. 2013, 162, 56–61.
- Robinson, D.T.; Caplan, M.; Carlson, S.E.; Yoder, R.; Murthy, K.; Frost, B. Early docosahexaenoic and arachidonic acid supplementation in extremely-low-birth-weight infants. Pediatr. Res. 2016, 80, 505–510.
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased Postnatal Docosahexaenoic and Arachidonic Acid Blood Levels in Premature Infants are Associated with Neonatal Morbidities. J. Pediatr. 2011, 159, 743–749.e2.
- Frost, B.L.; Jilling, T.; Caplan, M.S. The Importance of Pro-Inflammatory Signaling in Neonatal Necrotizing Enterocolitis. Semin. Perinatol. 2008, 32, 100–106.
- Caplan, M.S.; Jilling, T. The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids 2001, 36, 1053–1057.
- Moravec, A.R.; Siv, A.W.; Hobby, C.R.; Lindsay, E.N.; Norbash, L.V.; Shults, D.J.; Symes, S.J.K.; Giles, D.K. Exogenous Polyunsaturated Fatty Acids Impact Membrane Remodeling and Affect Virulence Phenotypes among Pathogenic Vibrio Species. Appl. Environ. Microbiol. 2017, 83, e01415-17.
- Koletzko, B.; Thiel, I.; Abiodun, P.O. The fatty acid composition of human milk in Europe and Africa. J. Pediatr. 1992, 120, S62–S70.
- Fidler, N.; Sauerwald, T.U.; Demmelmair, H.; Koletzko, B. Fat Content and Fatty Acid Composition of Fresh, Pasteurized, or Sterilized Human Milk. Adv. Exp. Med. Biol. 2001, 501, 485–495.
- Koletzko, B.; Agostoni, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological aspects of human milk lipids and implications for infant feeding: A workshop report. Acta Paediatr. 2011, 100, 1405–1415.
- Innis, S.M. Dietary Triacylglycerol Structure and Its Role in Infant Nutrition. Adv. Nutr. 2011, 2, 275–283.
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 28–40.
- Bobiński, R.; Bobińska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2020, 1–12.
- Cho, S.X.; Berger, P.J.; Nold-Petry, C.A.; Nold, M.F. The immunological landscape in necrotising enterocolitis. Expert Rev. Mol. Med. 2016, 18, e12.
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374.
- Drozdowski, L.A.; Clandinin, T.; Thomson, A.B.R. Ontogeny, growth and development of the small intestine: Understanding pediatric gastroenterology. World J. Gastroenterol. 2010, 16, 787–799.
- Innis, S.M.; Dai, C.; Wu, X.; Buchan, A.M.J.; Jacobson, K. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am. J. Physiol. Liver Physiol. 2010, 299, G1376–G1385.
- van Greevenbroek, M.M.; Voorhout, W.F.; Erkelens, D.W.; van Meer, G.; de Bruin, T.W. Palmitic acid and linoleic acid metabolism in Caco-2 cells: Different triglyceride synthesis and lipoprotein secretion. J. Lipid Res. 1995, 36, 13–24.
- van Greevenbroek, M.M.; van Meer, G.; Erkelens, D.W.; de Bruin, T.W. Effects of saturated, mono-, and polyunsaturated fatty acids on the secretion of apo B containing lipoproteins by Caco-2 cells. Atherosclerosis 1996, 121, 139–150.
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509.
- Gidding, S.S.; Lichtenstein, A.H.; Faith, M.S.; Karpyn, A.; Mennella, J.A.; Popkin, B.; Rowe, J.; van Horn, L.; Whitsel, L. Implementing American Heart Association pediatric and adult nutrition guidelines: A scientific statement from the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation 2009, 119, 1161–1175.
- Sodhi, C.P.; Fulton, W.B.; Good, M.; Vurma, M.; Das, T.; Lai, C.-S.; Jia, H.; Yamaguchi, Y.; Lu, P.; Prindle, T.; et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br. J. Nutr. 2018, 120, 665–680.
- Ng, D.H.C.; Klassen, J.R.L.; Embleton, N.D.; McGuire, W. Protein hydrolysate versus standard formula for preterm infants. Cochrane Database Syst. Rev. 2019.
- Walsh, V.; Brown, J.V.E.; Askie, L.M.; Embleton, N.D.; McGuire, W. Nutrient-enriched formula versus standard formula for preterm infants. Cochrane Database Syst. Rev. 2019.
- Qawasmi, A.; Landeros-Weisenberger, A.; Bloch, M.H. Meta-analysis of LCPUFA Supplementation of Infant Formula and Visual Acuity. Pediatrics 2012, 131, e262–e272.
- Willatts, P.; Forsyth, S.; Agostoni, C.; Casaer, P.; Riva, E.; Boehm, G. Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. Am. J. Clin. Nutr. 2013, 98, 536S–542S.
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; de Alencar, E.R. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812.
- Fusch, S.; Fusch, G.; Yousuf, E.I.; Rochow, M.; So, H.Y.; Fusch, C.; Rochow, N. Individualized Target Fortification of Breast Milk: Optimizing Macronutrient Content Using Different Fortifiers and Approaches. Front. Nutr. 2021, 8, 652641.
- Robinson, D.T.; Martin, C.R. Fatty acid requirements for the preterm infant. Semin. Fetal Neonatal Med. 2017, 22, 8–14.
- Lapillonne, A.; Jensen, C.L. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 143–150.
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global Standard for the Composition of Infant Formula: Recommendations of an ESPGHAN Coordinated International Expert Group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599.
- Mazzocchi, A.; D’Oria, V.; De Cosmi, V.; Bettocchi, S.; Milani, G.P.; Silano, M.; Agostoni, C. The Role of Lipids in Human Milk and Infant Formulae. Nutrients 2018, 10, 567.
- López-López, A.; López-Sabater, M.C.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargalló, A.I. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254.
- Koletzko, B.; Cheah, F.-C.; Domellof, M.; Poindexter, B.; Vain, N.; van Goudoever, J.P. Nutritional Care of Preterm Infants; Karger: Basel, Switzerland, 2021; Volume 122.
- Zou, L.; Pande, G.; Akoh, C.C. Infant Formula Fat Analogs and Human Milk Fat: New Focus on Infant Developmental Needs. Annu. Rev. Food Sci. Technol. 2016, 7, 139–165.
- Li, A.; Ha, Y.; Wang, F.; Li, W.; Li, Q. Determination of Thermally Induced trans-Fatty Acids in Soybean Oil by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy and Gas Chromatography Analysis. J. Agric. Food Chem. 2012, 60, 10709–10713.
- Caplan, M.S.; Russell, T.; Xiao, Y.; Amer, M.; Kaup, S.; Jilling, T. Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Intestinal Inflammation and Necrotizing Enterocolitis (NEC) in a Neonatal Rat Model. Pediatr. Res. 2001, 49, 647–652.
This entry is offline, you can click here to edit this entry!