1. Spontaneously Occurring Cancers in Companion Animals Represent a Unique Model for Human Cancers
The main goal of cancer research is to find new diagnostic and therapeutic anti-cancer strategies. Preclinical cancer research is mainly based on laboratory animal models with most of the studies performed on tumors grown in rodents [
1]. Laboratory in vitro and in vivo studies have been and remain an essential first step in cancer research. The main goal of preclinical research is to assess the toxicity and efficacy of new drugs prior to conducting human trials [
2]. Despite the common use of mice and other rodents in preclinical research, these models are poorly predictive of efficacy in human clinical trials [
3,
4]. Despite many successful laboratory animal studies, only 5–8% of new anticancer drugs are eventually approved for clinical use [
5,
6].
The lack of success in preclinical laboratory mice studies, both in xenograft and genetically engineered mice, could result from the failure to accurately reproduce the biological behavior and genetic and molecular background of the artificially induced cancer compared to a spontaneous human tumor, the inability to examine the specific tumor microenvironment and host characteristics, and a lack of the complexity and heterogenicity of naturally occurring cancers [
6,
7]. Experiments conducted in caged laboratory animals can be affected by high levels of stress that could affect the response to investigational drugs and immunotherapy. A lack of well-defined best practice protocols for the testing, treatment and procedures performed in laboratory animals compared to human clinical trials could also introduce bias [
8,
9].
Companion animals represent a unique model for human cancer for various reasons. Dogs and cats, unlike laboratory rodents, develop naturally occurring cancers that closely mimic the heterogenous nature of human tumors [
10,
11]. Many cancers arising in dogs and cats have similar clinical signs, appearances, and biological behavior to human cancers. Microscopic appearance and genetic and molecular background are also very similar in many types of cancers in dogs, cats, and people. The outbred characteristic in dogs, compared to studies in inbred laboratory animals, provides a background of genetic diversity that more closely parallels that of humans [
11,
12]. In addition, compared with the murine genome, the canine genome more closely resembles the human genome [
12,
13].
Cancer in pets, as in people, is one of the leading causes of death [
13,
14]. The life span of dogs and cats has increased in recent decades and now the incidence of cancer in dogs exceeds that of people, with around 40–50% of dogs older than 10 years dying of cancer [
15,
16]. Companion animals share all their lives with their owners, including the environmental and socioeconomic factors that predispose to cancer development [
17]. For example, obesity is considered one of the leading factors associate with increased cancer incidence, morbidity, and mortality in people [
18]. Obesity also affects dogs and cats. Around 50% of dogs are considered overweight [
19] and obese dogs are also more likely to have an owner that is obese [
20]. Companion animals and people are exposed to similar environmental risks factors, toxins, and carcinogens such as air pollution or pesticides in food and water [
21]. Spontaneous companion animal tumor models are likely to mimic the intricate and complex metabolic, genetic, and epigenetic alterations that are associated with cancer in people [
22].
Companion animals have a larger body size compared to rodents, allowing for easier and more frequent blood sampling for longitudinal assessment of drug efficacy/toxicity. Furthermore, the collection of larger biopsy samples compared to those of mice can be advantageous when multiple analyses are required [
23]. Identical imaging modalities can be applied to cancers in animals and humans (x-ray, CT, and MRI scan) so that any findings can be easily interpreted and compared. Responses to chemotherapy and drug toxicity in people is more comparable to those of companion animals than mice, and similar drugs are used to treat cancer in people and in pets [
23]. As an example, the CHOP chemotherapy protocol involving the use of cyclophosphamide, doxorubicin, vincristine, and prednisolone is used as a standard of care for the treatment of the most common type of lymphoma, diffuse large cell lymphoma (DLCL), in both dogs and human patients. Response rates and outcomes for some cancers (approximately one year survival in dogs translates to five years in people) are also comparable [
11]. However, many chemotherapy drugs are used at lower doses in pets compared to people as the main goal of treatment in veterinary oncology is to improve quality of life rather than attempting to achieve a cure [
24]. While conventional chemotherapy is used at lower doses in pets to avoid severe adverse events, target therapies and immunotherapies can often be used at similar doses in pets as in human patients [
25].
In the new era of cancer immunotherapy, companion animal models could play a key role in the testing and development of new treatment in people. The intricate crosstalk between the immune system and cancer is very difficult to replicate in artificially induced tumors in laboratory rodents. Dogs are exposed to a multitude of antigenic stimuli across their lifespan, including pathogenic and nonpathogenic bacteria, viruses, and parasites. Considering the large exposure of the intestine to numerous microbial antigens, it is not surprising that the intestinal microbiome can influence cancer growth and response to immunotherapy [
26,
27]. Dogs have a naturally developed intestinal microbiome that regulates the complex response to antigenic stimulation and diseases. Dogs are likely to respond to immunotherapy similarly to people, compared to laboratory rodents kept in disease free or minimal-disease conditions. Evaluation of the response to immunotherapy treatments and the longitudinal assessment of immune response parameters/biomarkers and side effects would be easier in companion animal than mice due to the easier collection of larger blood samples. Numerous studies in pets have assessed the relationship between cancer and the immune system; increased numbers of immunosuppressive regulatory T-cells (T-reg) have been found in various cancer types in dogs [
28,
29,
30]. Markers of potential response to immunotherapy such as programmed cell death 1 (PD-1) and its ligand, programmed cell death ligand 1 (PD-L1), have also been found to be overexpressed in cancer cells and cancer infiltrating lymphocytes in oral melanoma, lymphoma, osteosarcoma, and urothelial carcinoma in dogs [
31,
32,
33,
34,
35]. The first cancer immunotherapy vaccine has been successfully used in dogs with locally controlled oral melanoma and is now conditionally licensed in the USA [
36,
37]. Another new promising recombinant attenuated listeria monocytogenes vaccine expressing a chimeric human HER2 for HER-2+ osteosarcoma showed safety and efficacy in dogs and further studies are ongoing [
38].
In recent years, knowledge in veterinary medicine has grown significantly. Veterinarians can specialize in various medical disciplines including companion animal oncology. Qualified veterinary oncologists, like human oncologists, perform advanced treatments and follow best practice guidelines in performing clinical trials in pets. Veterinary oncologists, similarly to their medical colleagues, conduct clinical trials in dogs and cats with a strict standardized criteria for assessing grade of toxicity and tumor response [
39,
40], potentially offering preclinical data that are more precise, reliable, and more likely to be translated to successful human trials than the rodent models. Dogs and cats have a short life span and cancer progresses relatively quicker than in people, allowing for a more rapid collection of end-point data such as disease-free interval (DFI) and median survival time (MST) with significantly reduced cost compared to human clinical trials [
11].
Regulatory policies involving companion animal clinical trials are not always well defined and clear, but in general, the regulations are more flexible than in human clinical trials [
41]. As there are no clear international guidelines, rules often vary in different countries [
41]. However, it is often for the institution involved in the companion animal trial to set the rules when clear guidelines are not available [
42]. Clear and detailed owner consent forms and ethical approval from the institution involved in the study are usually the main requirements to perform a companion animal clinical trial in most countries [
42,
43].
Despite the advantages of using companion animal models, there are few limitations that need to be considered. The cancer heterogenicity of spontaneous companion animal models is an advantage, but also a disadvantage. When specific genetic/pathway alterations need to be studied, a more homogeneous and less diverse genetic background is preferable. Another limitation is cost; despite pet trials being cheaper than their human counterparts, they are still more expensive and more time consuming than rodent preclinical studies [
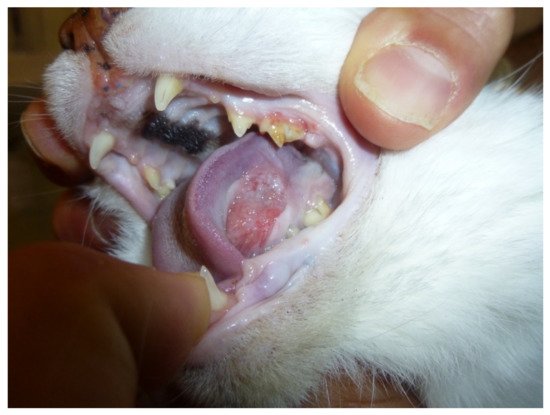
23]. Owner willingness to be enrolled in investigational studies and compliance with the terms and conditions of the trial are other potential disadvantages. Furthermore, an important factor that needs to be considered in pet trials is euthanasia. In many western countries, euthanasia in pets is widely recognized as a humane way to end life and so the subjective and personal decision of the owner to euthanize their pets could affect standardization of the MST. Despite the similarities of cancers in people and companion animals, there are species-specific differences in incidence as well as biological and clinical behavior that needs to be considered before choosing a specific companion animal cancer model. Feline oral squamous cell carcinoma and canine oral melanoma have previously been considered a good model for people suffering from head and neck carcinoma and mucosal melanoma, respectively [
17,
44]. An updated summary of the findings in these two types of tumors for translational cancer research, including new possible translational immunotherapies, will be discussed in more detail.