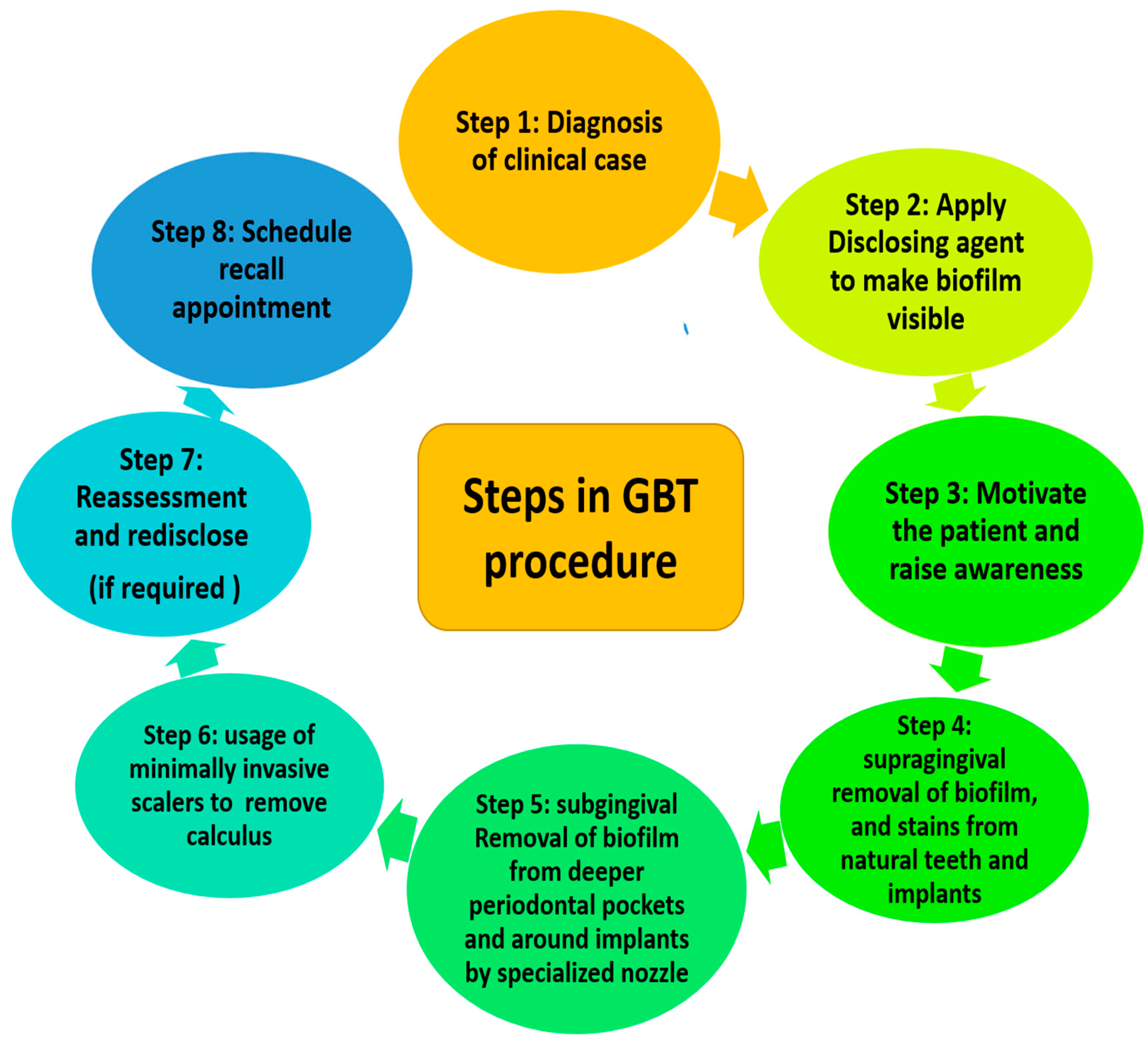
Guided biofilm therapy (GBT) is a new regimen where there is a sequential removal of plaque and calculus by initially detecting it with a disclosing agent followed by the usage of air abrasive powder for the removal of plaque and stains. Finally, the subgingival plaque and calculus are removed with a specialized nozzle and (if required) eventually scaling with a specialized tip is performed.
- biofilm
- air abrasives
- disclosing agents
- dental plaque
- periodontitis
- peri-implantitis
1. Introduction
Dental biofilm is a polymicrobial entity that resides on biotic and abiotic surfaces of the oral cavity [1]. These surfaces can range from hard or soft tissues of the oral cavity as well as the inanimate surfaces such as orthodontic bands, clear aligners, or prosthesis [2][3]. The supra and subgingival dental plaque biofilm can form on the tooth or implant surface. Being close to the gingival epithelium can deteriorate the periodontal and peri-implant health [3]. Dental plaque biofilms are also formed in some inaccessible regions of the oral cavity from where it is difficult to remove, thus compromising the home-care oral hygiene management. Although scaling and root planing (SRP) is considered the gold standard for mechanical plaque debridement [4], it also has its own disadvantages [5][6][7]. Nowadays, an alternative novel approach is being practiced for removing the biofilm by visualizing it with a disclosing agent and subsequently removing it with specialized air abrasive powder. Lastly, it is followed by the removal of supra and subgingival calculus using specialized instruments. This concept has been named guided biofilm therapy (GBT) [8].
2. Dental Biofilm and Its Relation to Periodontal and Peri-Implant Diseases
3. Rationale and Approaches for Non-Surgical Management of Dental Biofilm
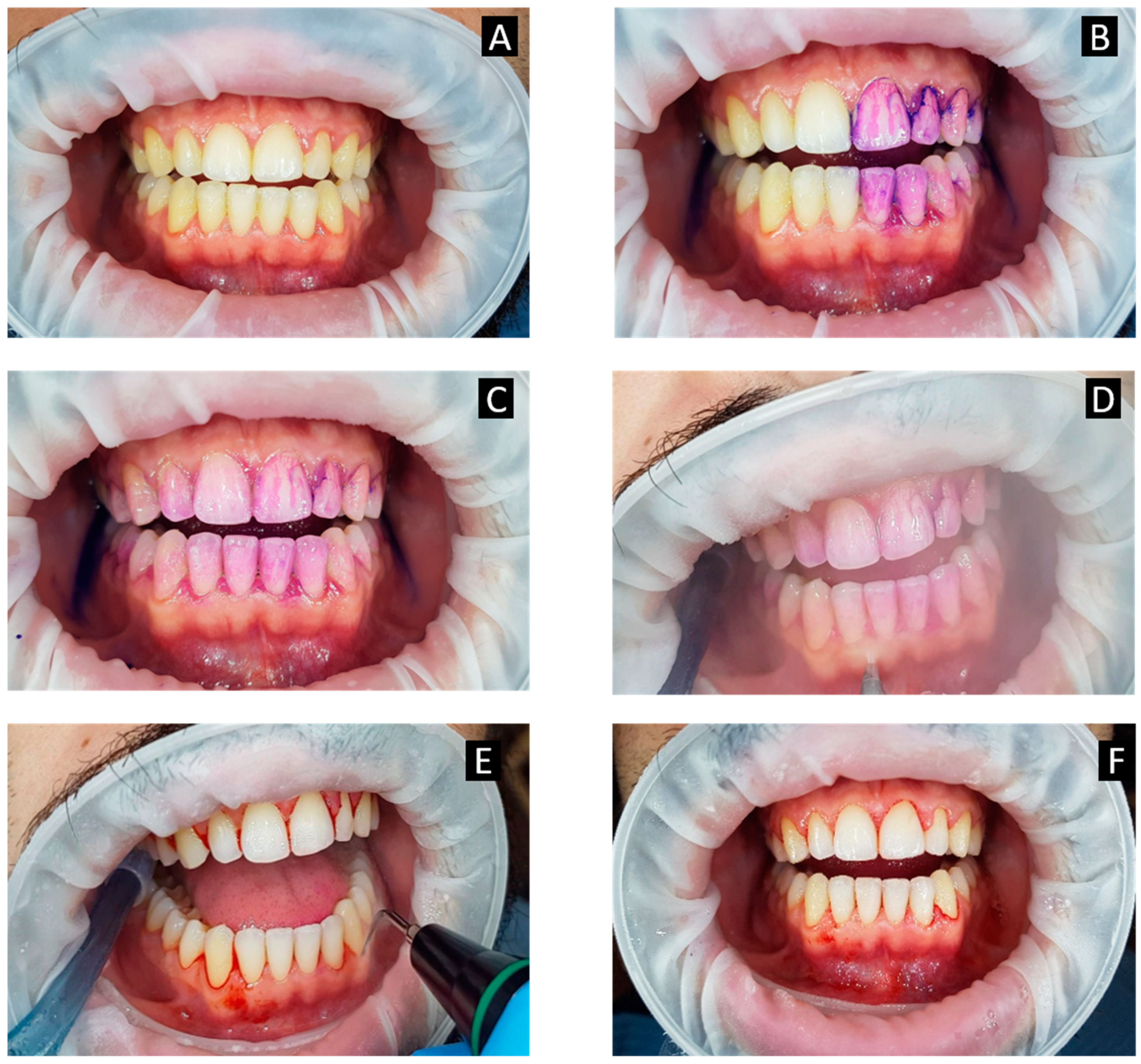
4. Role of Disclosing Agent
- (a) It should be used with caution on the restorative material as it can cause staining;
- (b) It should not be applied before the application of a sealant;
- (c) Solutions containing alcohol should not be stored for more than 2 to 3 months as the alcohol will evaporate, making the solution too concentrated;
- (d) Clinical assessments of soft tissue color, such as gingival status and gingival bleeding index, should be performed prior to the use of the plaque detector as dyeing the solution may mask the clinical status of the tissues;
- (e) Always assess for any kind of allergies of patients before using the detectors in any form [44].
6. Air Polishing Devices
7. Air Abrasive Powders
7.1. Sodium Bicarbonate (NaHCO₃)
7.2. Glycine Powder
7.3. Erythitol Powder
8. Guided Biofilm Therapy in Periodontal Disease and Peri-Implant Disease
| S. No | Author, Year | Objective | Subjects | Sample Size | Parameters | Outcome |
|---|---|---|---|---|---|---|
| 1 | Park, E.J. et al., 2018 [36] | Comparison of erythritol powder air-polishing device (EPAP) as a supplement to SRP therapy. | Human | Split mouth study design with twenty-one patients of moderate chronic periodontitis. | All patients received SRP (control) SRP+EPAP (test) on either jaw. Clinical and microbiological parameters were examined before treatment, 1 and 3 months post treatment. | Clinical parameters showed no significant difference between groups. However, counts of P. gingivalis were significantly lower in the test group at 1 month follow up period. Both parameters deteriorated at 3 months. |
| 2 | Caygur, A. et al., 2017 [37] | Comparison of glycine powder air-polishing (GPAP)combined with SRP in the treatment of periodontitis and halitosis. |
Human | Randomized clinical trial with sixty chronic periodontitis patients. | Patients were randomly allocated into control (SRP) and test group (SRP + GPAP). Clinical parameters were recorded at baseline and 1 month post treatment; also, the volatile sulphur compounds at baseline, immediately after treatment, and at 7, 14, and 30 days. | Clinical parameters were significantly reduced in both groups. The volatile sulphur compounds (VSCs) were significantly different at 1 month compared with baseline in both groups. GPAP has no additional benefit and is shown equally effective. |
| 3 | Hägi, T.T. et al., 2013 [38] | Comparison of erythritol powder by means of an air-polishing (EPAP) device and of (SRP) during SPT up to 3 months. | Human | Randomized clinical trial with forty patients on SPT, after completion of active treatment of moderate or severe periodontitis. | Patients were randomly assigned to control and test group. Clinical parameters such as plaque indices, BOP, PPD, and CAL were recorded at baseline and at 3 months. Patient’s comfort using a visual analog scale was also recorded. | All clinical parameters showed non-significant improvement. However, patients in test group showed significantly lower visual analogue scale (VAS) scores. |
| 4 | Müller, N. et al., 2014 [16] | Comparison of repeated subgingival air-polishing with a new erythritol powder containing 0.3% chlorhexidine with conventional ultrasonic debridement over 12 months. | Human | Randomized, parallel arm clinical trial with fifty patients on SPT. | Fifty patients were treated with subgingival air-polishing (test side) or ultrasonic debridement (control side) and were monitored at an interval of 3-month intervals up to 12 months. | Non-significant difference in clinical parameters was seen between the study groups. Test group showed significantly lesser count of A. actinomycetemcomitas at 12 months. |
| 5 | Reinhardt, B. et al., 2019 [9] | Comparison of periodontal pathogens of red complex after supragingival debridement (SD) with adjunctive full mouth (FM-GPAP) in periodontal healthy individuals. | Human | Randomized, split mouth study design with eighty-seven. | Subjects with 87 medically and periodontally healthy intraoral carriers of red complex bacteria were randomly assigned to receive SD with adjunctive FM-GPAP (test) or SD alone (control). Microbiological samples were obtained at baseline, and two, five, and nine days following intervention. | The count of red complex bacteria was significantly less in the test group in comparision to the control group following treatment and at day 9.However, the values were similar to baseline values when observed at 6 and 12 weeks. |
| 6 | Jentsch, H.F. et al., 2020 [10] | Comparison of adjunctive use of EPAPduring subgingival instrumentation (SI) with conventional NSPT. | Human | Randomized clinical trial with forty-two patients with moderate to severe periodontitis. | Patients were randomly assigned to control and test group receiving two different approaches of non-surgical periodontal therapy by SI, where test group additionally received EPAP. Clinical parameters, biomarkers and microorganism were measured at baseline, three and six months after SI. | Clinical parameters showed significant improvement at 2 and 6 months. However, test group showed more sites with PD ≥ 5 mm after six months. Significant reduction in the T. forsythia counts and T. denticola along with lesser values of matrix metalloprotienases -8 in the test group. |
| 7 | Hägi, T.T. et al., 2015 [12] | Clinical efficacy of low abrasive EPAP over a period of 6 months in patients undergoing SPT. | Human | Randomized clinical trial with forty chronic periodontitis patients. | Patients were randomly assigned to control (SRP) and test group (subgingival EPAP). Clinical parameters were evaluated at baseline, 3, and 6 month intervals. Site considered for evaluation had BOP with PPD of ≥ 4 mm |
A significant reduction of BOP, PPD and increase of CAL was observed between groups at 3 month intervals, but no significant difference at 6 months. No major change in periodontal pathogens recorded. |
| 8 | Tsang, Y.C. et al., 2018 [13] | Evaluation of GPAP as NSPT in subjects with chronic periodontitis. | Human | Randomized, split mouth study design with twenty-seven chronic periodontitis patients. | Patients received SRP and GPAP (test group) or SRP and air flushing with water (control group) at sites with PPD of ≥5 mm. Clinical parameters, gingival crevicular fluid(GCF) volumes, and the concentrations of interleukin-1β (IL-1β)and interleukin-1ra(IL-1ra) in GCF were measured at baseline and one, three, and six months after the intervention. | Significant improvements were recorded in clinical parameters in both groups. No significant difference in GCF levels of IL-1β and IL-1ra) were seen between the groups. |
| 9 | Kargas, K. et al., 2015 [14] | To evaluate the efficiency of subgingival GPAP during SPT. | Human | Randomized, split mouth study design with twenty-five chronic periodontitis patients. | Patients were randomly allocated to group receiving SRP with hand instruments, GPAP, subgingival ultrasonic debridement (UD), and no subgingival treatment (NT). Clinical parameters were recorded at baseline, three, and sixmonths. Subgingival samples were taken for microbiological analysis. | Clinically and microbiologicaly GPAP has no additional benefits over SRP or subgingival ultrasonic scaling. |
| 10 | Flemmig, T.F. et al., 2012 [15] | Comparison of supragingivally (GPAP) with conventional SRP in patients with in moderate-to-deep periodontal pockets. | Human | Randomized clinical trial with thirty patients with chronic periodontitis. | Patients were randomly allocated to received (FM- GPAP) or(SRP) followed by coronal polishing Patients rinsed with 0.12% chlorhexidine gluconate after debridement, and twice daily, for 2 weeks. | Test group showed significantly lesser total viable bacterial counts in chronic periodontitis patients when compared to SRP immediately after debridement and at the tenth day. |
| 11 | Wennström, J.L. et al., 2011 [17] | Comparison of subgingival air polishing (AP) compared with UD during SPT. | Human | Randomized, split mouth study design with twenty patients on SPT | Patients were randomly assigned two different subgingival debridement treatment groups—GPAP specially designed nozzle (test) and ultrasonic instrumentation (control). Clinical parameters and microbiological were recorded at baseline, fourteen, and sixtydays. | Results: both treatment procedures resulted in significant reductions in clinical parameters—BOP, PPD and relative attachment level at 2 months. Perceived treatment discomfort was less for AP than UD. |
| 12 | Solderer, A. et al. 2020 [26] | Comparison of mechanical debridement with/without air polishing on the healing of induced peri-implantitis. | Dogs | Non-randomized, animal study with forty-eight mandibular implants. | Depending on the study group, specific surgical cleaning approach is adopted along with augmentation procedure.
Histological measurements of the relative bone gain; depth of the defect, remaining bone, and soft tissue was measured. |
Non-significant partial regeneration was observed in all treatment approaches. However, pre-treatment with air polishing showed less inflammation. |
| 13 | Menini et al., 2019 [27] | Comparison of the cleaning efficacy of GPAP against two different professional oral hygiene techniques on implants supporting full-arch fixed prostheses. | Human | Randomized, split mouth study design with thirty patients with a total of 32 implant fixed full arch rehabilitations in the maxilla and/or mandible (134 implants). | Patients randomly assigned by following a splitmouth method: all the patients received glycine air polishing (G) in one side of the arch (n = 32), and sodium bicarbonate air polishing (B) (n = 16) or manual scaling with carbon-fiber curette (C) (n = 16) was performed in the opposite side. After the hygiene procedures, plaque index and spontaneous bleeding were recorded. | Plaque index reduction was significantly more for group treated with GPAP and sodium bicarbonate air polishing compared to manual scaling. Group treated with sodium bicarbonate were having maximum spontaneous bleeding as compared to other groups. It was concluded that the professional oral hygiene on implants using GPAP showed better patient acceptance and cleaning. |
| 14 | Siena et al., 2015 [28] | Comparative evaluation of professional oral hygiene with or without the adjunct of GPAP for the treatment of peri-implant mucositis | Human | Non-randomized clinical trial on 30 patients with peri implant mucositis | 30 patients were allocated into two groups. first group received professional oral hygiene manoeuvres (POH) while in the test group, received the GPAP.PPD, bleeding index (BI) and plaque index (PI) were measured at baseline, three, and six months. | The present reports showed that both techniques were useful for the treatment of peri-implant mucositis. In the test group (with glycine powder), a significant reduction inprobing depth was observed. |
| 15 | Lupi, S.M. et al. 2017 [29] | The study evaluated the efficacy of maintenance treatment with glycine powder on the periodontal health of peri-implant tissues. | Human | Single-masked, randomized clinical intervention trial on 46 patients with partial or total edentulism with 88 implants. | 46 patients with 88 implants were randomly assigned into two groups treated with either an air abrasive with the (GPAP) or to a manual debridement and chlorhexidine administration treatment group (MDA). Clinical data were collected at 0, 3, and 6 month intervals. PI, BOP, PPD, CAL, and bleeding score (BS) were analyzed. | Within the limits of the study, treatment with glycine seems appropriate in the maintenance of peri-implant health and more effective than the traditional treatment with plastic curette and chlorhexidine. |
| 16 | John, G. et al., 2015 [30] | Evaluation of the effectiveness of an air-abrasive device (AAD) for non-surgical treatment of peri-implantitis. | Human | Prospective, parallel grouped, randomized controlled clinical trial on twenty five patients with initial to moderate peri-implantitis. | 25 patients, with initial to moderate peri-implantitis in one implant, underwent an oral hygiene program and were randomly treated using either AAD (amino acid glycine powder) or mechanical debridement using carbon curettes and MDA. Clinical parameters were measured at baseline and tweleve months. | The present study has indicated that both treatment procedures resulted in comparable but limited CAL gains at 12 months. Furthermore, it could be detected that AAD was associated with significantly higher BOP decrease than MDA. Thus, AAD seems to be better than MDA. |
| 17 | Ji, Y.J. et al., 2102 [31] | This pilot clinical trial evaluated the effect of GPAP as an adjunct in treating peri-implant mucositis. | Human | Randomized clinical trial with twenty-four patients with peri-implant mucositis. | Twenty-four peri-implant mucositis patients were randomly assigned to test (12 subjects with 17 implants) and control (12 subjects with 16 implants) groups. In the test group, the sites with PPD of 4 mm were additionally treated by GPAP for 5 sec. Clinical parameters were measured at 1-week, one-month, and three-month recall visits. | At the 3-month visit, there was no significant difference existing between two groups in probing depth. This pilot clinical trial suggests that NSPT may be beneficial for treatment of peri-implant mucositis. However, adjunctive GPAP treatment seems to have a minimal beneficial effect. |
| 18 | Al Ghazal, L. et al., 2017 [32] | Comparing the two different methods of debridement for improving peri-implant soft tissue health for a follow up period of 12 months. | Human | Randomized, single blinded, parallel group clinical trial with twenty patients (25 implants. | 20 patients with no signs of pathologic bone loss around implants (25 implants) were selected. Patients were scheduled to be reviewed at 0, 3, 6, 9, and 12 months. Nine patients (15 implants) were randomly allocated to a test group (Air-FlowVR Perio, EMS) (AFP) and control group comprised of nine patients (10 implants) which were treated with titanium curettes (TC). Peri-implant GCF samples were analyzed to quantitatively measure the concentration of six interleukins. | The present study showed that both the treatment methods were effective in reducing perimplant inflammation with no difference in clinical parameter such as BOP. The present study showed a significant relationship between IL-6 and BOP. |
| 19 | Sahm, H. et al., 2011 [33] | To evaluate the effectiveness of an AAD for NST of peri-implantitis. | Human | Prospective, parallel group designed, randomized controlled clinical study with 30 patients of initial to moderate peri- implantitis. | Thirty patients, each of whom displayed at least one implant with initial to moderate peri-implantitis, were enrolled in an oral hygiene program (OHP) and randomly instrumented using either (1) AAD or (2) mechanical debridement using carbon curets and MDA. Clinical parameters were measured at baseline, 3, and 6 months after treatment [e.g., BOP, PPD, CAL]. | The present study concluded that both treatment procedures resulted in comparable but limited CAL gains at 6 months, and OHP+AAD was associated with significantly higher BOP reductions than OHP+MDA. |
| 20 | Persson, G.R.,et al., 2011 [34] | Clinical and microbiological NST of peri-implantitis lesions using either an erbium-doped: yttrium, aluminum, and garnet (Er:YAG) laser or an air-abrasive subgingival polishing method. | Human | Non-randomized clinical trial with 42 patients with peri-implantitis. | 42 patients with peri-implantitis were treated at one time with an Er:YAG laser or an air-abrasive device. Baseline and 6-month intraoral radiographs were assessed with a software program. The checkerboard DNA–DNA hybridization method was used to assess 74 bacterial species from the site with the deepest probing depth (PD) at the implant. | Non-significant probing depth reduction was seen in both the groups. No baseline differences in bacterial counts between groups were found. In the air-abrasive group, Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus anaerobius were found at lower counts at 1 month after therapy. Six-month data demonstrated that both methods failed to reduce bacterial counts. |
| 21 | Hentenaar, D.F. et al., 2021 [35] | Comparison of erythritol air polishing with piezoelectric ultrasonic scaling in the non-surgical treatment of peri-implantitis. | Human | Randomized clinical trial with eight patients of peri-implantitis having 139 implants. | 80 patients (n = 139 implants) with peri-implantitis PPD ≥5 mm, marginal bone loss (MBL) ≥2 mm as compared to bone level at implant placement, bleeding, and/or suppuration on probing (BOP/SOP)) were randomly allocated to EPAP or ultrasonic treatment. Clinical outcome and pain/discomfort VAS were measure at 0, 3,6,9,12 months. | Three months after therapy, no significant difference in mean BOP, plaque score, PPD, MBL between the EPAP and ultrasonic group. Pain/discomfort was low in both groups. EPAP seems as effective as piezoelectric ultrasonic scaling in the NST of peri-implantitis. |
-
The use of a plaque disclosing agent allows the operator to determine the patient compliance in executing proper oral hygiene practices. It also allows the patient to visualize areas that were neglected;
-
The use of an air-polishing device can remove the disclosed plaque effectively and safely without causing soft tissue damage compared to conventional rubber cups, especially during subgingival plaque removal;
-
The removal of plaque using air polishing prior to ultrasonic scaling provides better visible access to calculus deposits. Instead of the indiscriminate use of ultrasonic scalers for the entire dentition, the operator can now target the use of ultrasonic scalers on sites with mineralized deposits. This minimizes soft tissue damage and CAL caused by ultrasonic scaling at sites with shallow pocket depths. From the patient’s perspective, this translates to lesser discomfort and sensitivity experienced during ultrasonic scaling. Overall, treatment time is also reduced;
-
A second plaque disclosure provides quality control and assurance to the patient as well as the operator.
9. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9091966
References
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. Available online: https://europepmc.org/article/MED/22319749 (accessed on 15 August 2021).
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S12–S22.
- Nimbulkar, G.; Garacha, V.; Shetty, V.; Bhor, K.; Srivastava, K.C.; Shrivastava, D.; Sghaireen, M.G. Microbiological and Clinical evaluation of Neem gel and Chlorhexidine gel on Dental Plaque and Gingivitis in 20–30 Years Old Adults: A Randomized Parallel-Armed, Double-Blinded Controlled Trial. J. Pharm. Bioallied. Sci. 2020, 12 (Suppl. 1), S345–S351.
- Larsen, T.; Fiehn, N.E. Dental biofilm infections-an update. APMIS 2017, 125, 376–384.
- Rode Sde, M.; Gimenez, X.; Montoya, V.C.; Gómez, M.; Blanc, S.L.; Medina, M.; Salinas, E.; Pedroza, J.; Zaldivar-Chiapa, R.M.; Pannuti, C.M.; et al. Daily biofilm control and oral health: Consensus on the epidemiological challenge-Latin American Advisory Panel. Braz. Oral Res. 2012, 26 (Suppl. 1), 133–143.
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 24.
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802.
- Shrivastava, D.; Srivastava, K.C.; Ganji, K.K.; Alam, M.K.; Al Zoubi, I.; Sghaireen, M.G. Quantitative Assessment of Gingival Inflammation in Patients Undergoing Nonsurgical Periodontal Therapy Using Photometric CIELab Analysis. BioMed Res. Int. 2021, 30, 2021.
- Shrivastava, D.; Srivastava, K.C.; Dayakara, J.K.; Sghaireen, M.G.; Gudipaneni, R.K.; Al-Johani, K.; Baig, M.N.; Khurshid, Z. BactericidalActivity of Crevicular Polymorphonuclear Neutrophils in Chronic Periodontitis Patients and Healthy Subjects under the Influence of Areca Nut Extract: An In Vitro Study. Appl. Sci. 2020, 10, 5008.
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419.
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725.
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490.
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355.
- Amano, A. Host-parasite interactions in periodontitis: Microbial pathogenicity and innate immunity. Periodontology 2000 2010, 54, 9–14.
- Kajiya, M.; Kurihara, H. Molecular Mechanisms of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 930.
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11.
- Dhir, S. Biofilm and dental implant: The microbial link. J. Indian Soc. Periodontol. 2013, 17, 5–11.
- Mombelli, A.; Lang, N.P. Microbial aspects of implant dentistry. Periodontology 2000 1994, 4, 74–80.
- Sahni, K.; Khashai, F.; Forghany, A.; Krasieva, T.; Wilder-Smith, P. Exploring Mechanisms of Biofilm Removal. Dentistry (Sunnyvale) 2016, 6, 371.
- Fatima, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208.
- Ng, E.; Byun, R.; Spahr, A.; Divnic-Resnik, T. The efficacy of air polishing devices in supportive periodontal therapy: A systematic review and meta-analysis. Quintessence Int. 2018, 49, 453–467.
- Renvert, S.; Persson, G.R. Supportive periodontal therapy. Periodontology 2000 2004, 36, 179–195.
- Cortés-Acha, B.; Figueiredo, R.; Seminago, R.; Roig, F.J.; Llorens, C.; Valmaseda-Castellón, E. Microbiota Analysis of Biofilms on Experimental Abutments Mimicking Dental Implants: An In Vivo Model. J. Periodontol. 2017, 88, 1090–1104.
- Schultz-Haudt, S.; Bruce, M.A.; Bibby, B.G. Bacterial factors in nonspecific gingivitis. J. Dent. Res. 1954, 33, 454–458.
- Loesche, W.J. Clinical and microbiological aspects of chemotherapeutic agents used according to the specific plaque hypothesis. J. Dent. Res. 1979, 58, 2404–2412.
- Meto, A.; Colombari, B.; Odorici, A.; Giva, L.B.; Pericolini, E.; Regina, A.L.; Blasi, E. Antibacterial Effects of MicroRepair® BIOMA-Based Toothpaste and Chewing Gum on Orthodontic Elastics Contaminated In Vitro with Saliva from Healthy Donors: A Pilot Study. Appl. Sci. 2020, 10, 6721.
- Park, E.J.; Kwon, E.Y.; Kim, H.J.; Lee, J.Y.; Choi, J.; Joo, J.Y. Clinical and microbiological effects of the supplementary use of an erythritol powder air-polishing device in non-surgical periodontal therapy: A randomized clinical trial. J. Periodontal. Implant Sci. 2018, 48, 295–304.
- Fleischer, H.C.; Mellonig, J.T.; Brayer, W.K.; Gray, J.L.; Barnett, J.D. Scaling and root planing efficacy in multirooted teeth. J. Periodontol. 1989, 60, 402–409.
- Rabbani, G.M.; Ash, M.M., Jr.; Caffesse, R.G. The effectiveness of subgingival scaling and root planing in calculus removal. J. Periodontol. 1981, 52, 119–123.
- Eaton, K.A.; Kieser, J.B.; Davies, R.M. The removal of root surface deposits. J. Clin. Periodontol. 1985, 12, 141–152.
- Fischer, C.; Wennberg, A.; Fischer, R.G.; Attström, R. Clinical evaluation of pulp and dentine sensitivity after supragingival and subgingival scaling. Endod. Dent. Traumatol. 1991, 7, 259–265.
- Sultan, D.A.; Hill, R.G.; Gillam, D.G. Air-polishing in subgingival root debridement: A critical literature review. J. Dent. Oral Biol. 2017, 2, 1065. Available online: https://www.gavinpublishers.com/article/view/air-polishing-in-subgingival-root-debridement-during-supportive-periodontal-care-a-review (accessed on 15 August 2021).
- Greenstein, G. Periodontal response to mechanical non-surgical therapy: A review. J. Periodontol. 1992, 63, 118–130.
- Boyd, L.D.; Mallonee, L.F.; Wyche, C.J.; Halaris, J.F. Wilkins’ Clinical Practice of the Dental Hygienist, 13th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2016.
- Montevecchi, M.; Checchi, V.; Gatto, M.R.; Klein, S.; Checchi, L. The use of a disclosing agent during resective periodontal surgery for improved removal of biofilm. Open Dent. J. 2012, 6, 46–50.
- Nepale, M.B.; Varma, S.; Suragimath, G.; Abbayya, K.; Zope, S.; Kale, V. A prospective case-control study to assess and compare the role of disclosing agent in improving the patient compliance in plaque control. J. Oral Res. Rev. 2014, 6, 45.
- Barrows, J.N.; Lipman, A.L.; Bailey, C.J. Color additives: FDA’s regulatory process and historical perspectives. Food Saf. Mag. 2003, 1. Available online: https://www.food-safety.com/articles/4207-color-additives-fdas-regulatory-process-and-historical-perspectives (accessed on 15 August 2021).
- Allam, K.V.; Kumar, G.P. Colorants-the cosmetics for the pharmaceutical dosage forms. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. 3), 9.
- Datta, D.; Kumar, S.R.; Narayanan, A.; Selvamary, A.L.; Sujatha, A. Disclosing solutions used in dentistry. World J. Pharm. Res. 2017, 6, 1648–1656.
- Block, P.L.; Lobene, R.R.; Derdivanis, J.P. A two-tone dye test for dental plaque. J. Periodontol. 1972, 43, 423–426.
- Checchi, L.; Forteleoni, G.; Pelliccioni, G.A.; Loriga, G. Plaque removal with variable instrumentation. J.Clin. Periodontol. 1997, 24, 715–717.
- Tan, A.E.; Wade, A.B. The role of visual feedback by a disclosing agent in plaque control. J. Clin. Periodontol. 1980, 7, 140–148.
- Petersilka, G.J. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontology 2000 2011, 55, 124–142.
- Mensi, M.; Scotti, E.; Sordillo, A.; Agosti, R.; Calza, S. Plaque disclosing agent as a guide for professional biofilm removal: A randomized controlled clinical trial. Int. J. Dent. Hyg. 2020, 18, 285–294.
- Petersilka, G.J.; Schenck, U.; Flemmig, T.F. Powder emission rates of four air polishing devices. J. Clin. Periodontol. 2002, 29, 694–698.
- Donnet, M.; Fournier, M.; Schmidlin, P.R.; Lussi, A. A Novel Method to Measure the Powder Consumption of Dental Air-Polishing Devices. Appl. Sci. 2021, 11, 1101.
- Prof, Û.; Nardi, G.M. Système d’aéro-POLISSAGE COMBI Touch. Available online: www.mectron.fr ou (accessed on 15 August 2021).
- Momber, A.; Kovacevic, R. Principles of Abrasive Water Jet Machining; 9.6; Springer: New York, NY, USA, 1998.
- Barnes, C.M. The management of aerosols with airpolishing delivery systems. J. Dent. Hyg. 1991, 65, 280–282.
- Barnes, C.M. An In-Depth Look at Air Polishing; University of Nebraska Medical Center: Omaha, NE, USA, 2010.
- Conserva, E.; Pisciotta, A.; Bertoni, L.; Bertani, G.; Meto, A.; Colombari, B.; Blasi, E.; Bellini, P.; de Pol, A.; Consolo, U.; et al. Evaluation of biological response of STRO-1/c-Kit enriched human dental pulp stem cells to titanium surfaces treated with two different cleaning systems. Int. J. Mol. Sci. 2019, 20, 1868.
- Meto, A.; Conserva, E.; Liccardi, F.; Colombari, B.; Consolo, U.; Blasi, E. Differential efficacy of two dental implant decontamination techniques in reducing microbial biofilm and re-growth onto titanium disks in vitro. Appl. Sci. 2019, 9, 3191.
- Munro, I.C.; Berndt, W.O.; Borzelleca, J.F.; Flamm, G.; Lynch, B.S.; Kennepohl, E.; Bär, E.A.; Modderman, J. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem. Toxicol. 1998, 36, 1139–1174.
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonasgingivalis. Mol. Oral Microbiol. 2013, 28, 435–451.
- Caygur, A.; Albaba, M.R.; Berberoglu, A.; Yilmaz, H.G. Efficacy of glycine powder air-polishing combined with scaling and root planing in the treatment of periodontitis and halitosis: A randomized clinical study. J. Int. Med. Res. 2017, 45, 1168–1174.
- Hägi, T.T.; Hofmänner, P.; Salvi, G.E.; Ramseier, C.A.; Sculean, A. Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive periodontal therapy: A randomized, controlled clinical study. Quintessence Int. 2013, 44, 753–761.
- Müller, N.; Moëne, R.; Cancela, J.A.; Mombelli, A. Subgingival air-polishing with erythritol during periodontal maintenance: Randomized clinical trial of twelve months. J. Clin. Periodontol. 2014, 41, 883–889.
