1. Introduction
Several viruses have the ability to hijack pre-existing mechanisms of cellular communication to facilitate direct cell-to-cell viral spread [1,2,3], and the human immunodeficiency virus type 1 (HIV-1) is not an exception [1,4]. Before the definition of the precise mechanisms of cell-to-cell viral transmission, early studies highlighted the increased efficiency of HIV-1 spread by cellular contacts as compared to the diffusion-limited movement of free viral particles, suggesting that cell-to-cell dissemination might be up to 1000 times more efficient [5]. However, the first detailed description of a stable cellular junction between infected and non-infected cells to facilitate viral spread, known as virological synapse (VS), was reported for the human T cell leukaemia virus type 1 (HTLV-1), which is inefficient at infecting T cells and requires cellular contacts for effective spread [6]. Soon after this description, several studies showed co-clustering of HIV-1 proteins with their receptors CD4 and CXCR4, together with a massive viral transmission at the stable interface formed between HIV-1-infected and non-infected CD4+ T cells [7,8], thus expanding the concept of VS to HIV-1.
In addition to the VS, there is another type of synapse formed between antigen presenting cells (APCs) such as DCs and CD4+ T cells, which can even operate in the absence of productive infection of the donor APC. During antigen presentation, the formation ofcognate DC:T cell conjugates or ‘immunological synapses’ is necessary for the activation of T cells [30,31]. Once activated, T cells proliferate and differentiate into effector cells, which mediate adaptive immune responses aimed to eliminate invading viruses [32]. Intriguingly, upon HIV-1 infection, the intimate cell-to-cell contacts formed between DCs and CD4+ T cells can boost viral transmission via the formation of an ‘infectious synapse’ [33] that allows for systemic HIV-1 dissemination. Here we focus on how DCs, which are the most potent APCs found in our organism [34,35], are also the ones with greater capacity to boost HIV-1 transmission via a cell-to-cell transfer mechanism.
2. Breaking the Ice: DCs Orchestrate Immune Responses against HIV-1 and Other Viruses
DCs act as pivotal players in the initiation of immunity against invading viruses [
36,
37], participating in both innate and adaptive immune responses. These cellular sentinels patrol distinct mucosae and, upon infection, viral sensing triggers rapid innate immune responses to contain viral spread. DC activation also elicits cellular migration towards secondary lymphoid tissues, where DCs acquire a fully mature phenotype and become competent for presenting antigens to T cells and activate them [
34,
35].
DCs form an integral part of innate immunity, along with other leukocytes and tissue-resident cells. DCs are present at the sites of pathogen invasion such as mucosal surfaces and the skin, and are among the first cells encountering these pathogens. DCs detect molecular patterns shared by broad groups of pathogens, termed pathogen-associated molecular patterns (PAMPs), which include viral RNA or DNA genomes, bacterial lipopolysaccharide (LPS) and yeast mannans [
38,
39]. DCs recognize these conserved motifs through pattern-recognition receptors (PRRs) [
40]. A well-studied family of PRRs are Toll-like receptors (TLRs), which recognize a variety of ligands [
41,
42], each TLR having a particular sub-cellular localization and ligand specificity [
43]. For instance, endosomal TLR7 and TLR8 recognize single-stranded RNA, while TLR9 binds DNA, and TLR4 recognizes LPS, an integral component of the outer membrane of gram-negative bacteria. Another group of PRRs found on DCs are C-type lectin receptors (CLRs), which include DC-SIGN (CD209), L-SIGN (CD299, Clec4M) and LSECtin (Clec4G), and recognize high mannose-containing glycans [
44,
45]. Within the group of I-type lectin receptors, the sialic acid-binding Ig-like lectins (Siglecs) are the best characterized members [
46,
47]. They are expressed by DCs, macrophages and monocytes and recognize sialic acids found on pathogens and also in host cells [
48].
Viral recognition by DCs triggers the expression of genes involved in the secretion of cytokines and chemokines [
49,
50], which create a proinflammatory environment to eliminate or limit its replication. The main antiviral cytokines are type I interferons (IFNs), such as IFNα and IFNβ, and plasmacytoid DCs are major producers of these cytokines [
51]. DCs that patrol mucosal surfaces display an immature status and can trap viruses at the entry sites, degrade them in endosomal lytic compartments and load pathogen-derived peptides onto molecules of the major histocompatibility complex (MHC). When this occurs, DCs become activated and migrate to the secondary lymphoid tissues [
52], where DCs present viral-derived antigens to naïve T lymphocytes.
There are different ways of antigen presentation by DCs to T cells, depending partially on the origin of such antigens. Endogenous antigens are those expressed by the DC itself (for example viral proteins synthesized in the cytoplasm upon viral infection), and after proteasomal cleavage, the derived peptides are loaded onto MHC class I molecules and presented to CD8
+ T cells [
53]. In contrast, exogenous antigens are internalized by DCs through pinocytosis, phagocytosis and receptor-mediated endocytosis, processed by endosomal proteases, and the derived peptides are incorporated onto MHC class II molecules that also reach the cell surface [
54]. MHC-II:peptide complexes are recognized by CD4
+ T cells, which differentiate into several effector cell subtypes. In the context of viral infection these cells are mainly Th1 and T follicular helper cells [
55], which prompt specific antiviral responses.
Of note, DCs have the unique capacity of presenting exogenous antigens to CD8
+ T cells via MHC-I, a process known as ‘cross-presentation’ [
56]. This mechanism allows antigen presentation to CD8
+ T cells without productive DC infection, and is an efficient presentation pathway for viruses such as influenza A virus (IAV) [
57,
58] and HIV-1 [
59]. Another non-classical antigen presentation pathway is that followed by endogenous peptides from measles virus [
60], IAV [
61] and HIV-1 [
62], which are loaded onto MHC-II molecules instead of MHC-I molecules, being therefore presented to CD4
+ T cells.
Despite the fined-tuned machinery for antigen presentation displayed by DCs, DC:T cell conjugates also represent a unique niche for viral transmission through the formation of infectious synapses [
33], a mechanism extensively studied for HIV-1, that is also hijacked by other enveloped viruses.
3. When Immunity Is Put on Ice: DCs as Promoters of HIV-1 Cell-to-cell Transmission
Although DCs orchestrate key innate and adaptive immune antiviral responses [
36,
37,
63], HIV-1 and other viruses have evolved strategies to evade DC surveillance [
64,
65,
66]. Indeed, viruses exploit DC function as a way to fuel infection of target cells, colonizing distant tissues as DCs migrate (
Figure 1). Landmark studies carried out in the 90s in the laboratory of Ralph Steinman showed that the efficacy of HIV-1 infection of CD4
+ T cells was increased when DCs were added in co-culture as compared to the transmission of cell-free viruses [
67,
68]. Noteworthy, DCs are non-permissive to HIV-1 infection, as they express low levels of viral receptor and co-receptors [
69], efficiently degrade incoming viruses [
70,
71] and express several restriction factors such as SAMHD1 that interfere with viral replication [
72,
73,
74,
75,
76,
77]. However, these pioneering studies demonstrated that DCs can transmit a vigorous HIV-1 infection to bystander CD4
+ T cells in the absence of productive viral replication on DCs, a mechanism of viral cell-to-cell transmission known as
trans-infection [
44,
67].
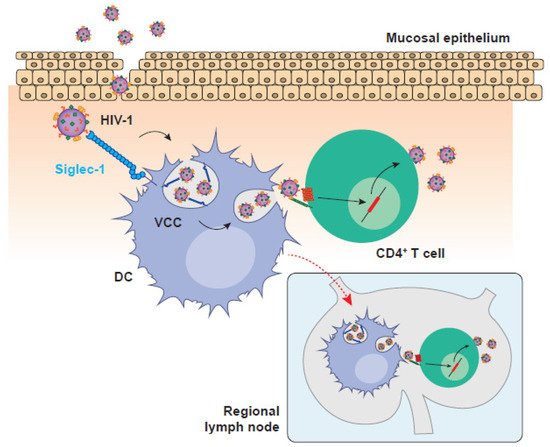
Figure 1. HIV-1 invasion is boosted by DC-mediated viral transmission in the mucosa and the migration to secondary lymphoid tissues. HIV-1 replication in the mucosa is facilitated by Siglec-1-expressing DCs that can mediate viral transmission to mucosal CD4+ T cells or migrate to secondary lymphoid tissues where the interaction with other target CD4+ T cells accelerates the settlement of systemic infection. HIV-1: human immunodeficiency virus type 1; VCC: viral containing compartment; DC: dendritic cell.
Trans-infection is one of the most potent viral transmission processes identified so far, but is only boosted when DC infection is restricted, as it is the case of HIV-1.
Trans-infection was initially attributed to the activity of a DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), a C-type lectin receptor expressed by DCs that recognizes the HIV-1 envelope glycoprotein [
44,
78]. However, several studies suggested that other receptors aside from DC-SIGN operated in HIV-1 transmission [
79,
80,
81,
82,
83,
84,
85,
86]. This was suspected because DC maturation greatly increased HIV-1
trans-infection capacity while it decreased the expression of DC-SIGN [
86], and because antibodies directed against DC-SIGN were not able to consistently block HIV-1 transmission [
82]. Such inconsistencies led to the identification, almost a decade ago, of the sialic acid-binding immunoglobulin-like lectin 1 (Siglec-1/CD169) as the key molecule for DC-mediated HIV-1
trans-infection [
87,
88].
Siglec-1, also termed sialoadhesin, is an I-type lectin expressed by APCs of myeloid origin such as DCs, macrophages and monocytes [
87,
88,
89,
90,
91]. At a structural level, this receptor consists of different immunoglobulin-like domains or ‘sets’, all of them extracellular. The N-terminal V-set domain contains the ligand binding activity, while the 16 extracellular C2-set domains project the V-set domain out of the cell glycocalyx, allowing for the interaction with extracellular molecules [
48,
92,
93]. Siglec-1 has affinity for sialic acid present in both N- and O-glycans, with a higher preference for α2-3 linkages [
94]. These sugars are found in a variety of complex glycolipid molecules such as gangliosides GM1a and GM3, which are components of the cell and viral membranes. In particular, these gangliosides are present in the membrane of HIV-1, allowing for viral binding to DCs via Siglec-1 and the subsequent transmission to by-stander CD4
+ T cells [
87,
88,
95,
96]. Siglec-1 avidity for sialylated ligands is increased upon clustering of thousands of gangliosides in the viral membrane [
48].
HIV-1
trans-infection is a dynamic process that involves viral attachment to Siglec-1, internalization within a viral containing compartment (VCC), and viral release to the intercellular space during the formation of DC:CD4
+ T cell infectious synapses [
25,
97]. Following Siglec-1 recognition, HIV-1 particles concentrate on the surface of DCs [
14,
98] and are internalized into non-classical and non-acidic endosomal VCC enriched in tetraspanins, MHC-II and Siglec-1 [
89,
97,
99]. Of note, VCCs and their content remain connected to the extracellular milieu [
14,
98,
99], which facilitates the transmission of trapped HIV-1 particles upon the formation of DC:CD4
+ T cell contacts. Although the physiological function of VCCs remains unclear, it might be related to antigen dissemination and storage, as this compartment also serves as a depot of antigen-containing extracellular vesicles that are also captured by Siglec-1 and can prime adaptive immune responses [
13,
100,
101]. Therefore, HIV-1 exploits a pre-exiting Siglec-1-dependent antigen dissemination pathway to gain access to target CD4
+ T cells.
Aside from subverting antigen presentation, HIV-1 also exploits DC migratory capacity to spread systemically. This has led to the idea that DCs can operate as ‘Trojan Horses’ and disseminate HIV-1 from the portals of viral entry to lymphoid tissues [
67,
102]. HIV-1 is mainly acquired through sexual transmission [
103] and early events of retroviral infection have been extensively studied in non-human primate models. Following early replication at the reproductive mucosa, DCs bearing retroviruses can be found in draining lymph nodes of different non-human primate models as soon as 24 h after vaginal challenge [
104,
105,
106,
107]. Noteworthy, viral spread does not only rely on the productive infection of DCs, but also on the transference of captured viral particles via
trans-infection [
108,
109,
110,
111]. Indeed, through the ex vivo culture of cells derived from human cervical tissues, we demonstrated that this mechanism relies on Siglec-1 [
112]. Of note, we identified the presence of Siglec-1-enriched VCCs in the biopsy of a viremic HIV-1
+ patient [
112], indicating that cervical DC-mediated HIV-1
trans-infection might be a relevant process for viral acquisition in vivo. Thus,
trans-infection may be key to establishing HIV-1 infection in the mucosa, leading to systemic viral dissemination thanks to the migratory capacity of DCs.
To sum up, DCs are key immune cells for the establishment of adaptive immune responses due to their migratory and antigen presentation capacities. However, pathogens such as HIV-1 have evolved strategies to subvert this immune function to efficiently spread through DC-mediated cell-to-cell contacts. Future studies should tackle this mechanism of viral dissemination, which could lead to the generation of new therapeutic strategies against HIV-1 infection.