Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Endometrial receptivity plays a crucial role in fertilization as well as pregnancy outcome in patients faced with fertility challenges. The optimization of endometrial receptivity may help with normal implantation of the embryo, and endometrial receptivity may be affected by numerous factors.
- polyunsaturated fatty acids
- endometrial receptivity
- decidualization
- prostaglandins
- estrogen
- progesterone
1. Introduction
Pregnancy is thought to result from the interaction of local secretory factor-mediated embryonic developmental capacity and endometrial receptivity [1]. Therefore, impaired or decreased endometrial receptivity may result in the failure of embryo implantation [2]. Implantation failure is a problem in assisted reproductive technology (ART) that remains unresolved and has raised concerns in the scientific community [3,4]. In Europe, the overall number of ART cycles has continued to increase annually [5]. With scientific and technological advancements, ART has made great progress in terms of embryo selection and cryopreservation technology, but treatment still fails in numerous patients who cannot conceive.
It is well known that the endometrium has a lipid component that is very important for reproduction. Triglycerides and eicosanoids are among the lipid mediators secreted from the endometrium. From the eicosanoid family, prostaglandins (PGs), thromboxanes, leukotrienes, endocannabinoids and sphingolipids have been found to play a role in reproduction [6,7,8]. Fatty acids are divided into three categories, namely monounsaturated, polyunsaturated and saturated fatty acids. Polyunsaturated fatty acids (PUFAs) contain multiple double bonds, and they can be classified into the following three categories according to their double bond positions: n-3, n-6 and n-9 fatty acids. In addition, PUFAs include long-chain unsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [9,10,11].
These fatty acids play an important role in human growth and development, such as body growth, visual system formation and human reproduction [12]. Linoleic acid is a PUFA commonly found in the daily diet and is derived from vegetable oils such as safflower, sunflower and canola oil [12]. The majority of n-3 unsaturated fatty acids are derived from Alpha [α]-linolenic acid and are found in the chloroplasts of green leafy vegetables. These two important fatty acids can be converted to long-chain unsaturated fatty acids in the liver through desaturation and elongation enzyme systems. In addition to dietary sources, for women of childbearing age, taking PUFA supplements may be beneficial for the treatment of menstrual dysfunction [13]. In animal experiments, researchers found that the addition of n-3 polyunsaturated fatty acids to the feed of heifers improved reproductive efficiency, and the study showed that n-3 polyunsaturated fatty acids had a positive effect on the expression of key genes during the window of implantation [14]. Lipid metabolism is required for uterine receptivity and embryo implantation [15].
2. PUFAs, Sex Hormones and Endometrial Receptivity
2.1. Estrogen
The endometrium is very sensitive to hormone changes, especially in the presence of steroid hormones, and this change prepares the endometrium for embryonic implantation and decidualization [68]. Estrogen and progesterone are critical mediators in embryo implantation and decidualization [36]. Estrogen, in particular, plays an important role during the embryo implantation window, and high levels of estrogen can cause the implantation window to close [69].
It appears that n-3 PUFAs are associated with estradiol. However, two studies investigating the relationship between maternal intake of n-3 and n-6 PUFAs during pregnancy and estradiol levels did not provide definitive results [70,71].
It was found that, in the guinea pig estrous cycle, free PUFA patterns in the plasma may affect steroid hormone concentrations [70]. ALA-rich diets were shown to increase follicular estradiol concentrations in cows, while both LA and ALA decreased progesterone concentrations in the luteal phase [72]. Compared to n-6 PUFAs, n-3 PUFAs were also shown to increase progesterone concentrations in sheep follicles, while estrogen was not affected [68]. These effects are mediated by altering PG synthesis and steroidogenesis, which may be differentially affected by n-3 and n-6 PUFAs [36]. In addition, the effects of these fatty acids play different roles between oestrus and dioestrus. Another study found that DHA stimulated bovine granulosa cell proliferation and steroidogenesis [69]. The positive effect of PUFAs on the rate of sex hormone secretion during the follicular phase may be due to enlarging follicles, thus producing more steroid hormones; overall, it can be hypothesized that PUFAs increased steroid production or altered PG synthesis [73]. Similarly, n-3 long-chain PUFAs were found to affect gene expression as well as estrogen metabolism [74].
2.2. Progesterone
Progesterone is an important steroid hormone in the body, and it is also known as the pregnancy hormone due to its important role during that period. During the first week of pregnancy, progesterone is mainly derived from the corpus luteum and, subsequently, as the gestational weeks progress, progesterone is mainly synthesized by the placenta [75]. The mitochondria in the syncytial trophectoderm are the main sites of the synthesis of this steroid hormone, which is synthesized via a two-step reaction [76].
2.3. Estrogen and Progesterone Signaling in Endometrial Receptivity
Estrogen and progesterone act mainly through estrogen receptor (ER) and PR, which are both nuclear receptors. It was found that the proliferation of endometrial epithelial cells is mainly achieved by estrogen acting on ERs [77]. ERs in epithelial and stromal cells play an important role in the formation of endometrial receptivity, as well as in epithelial differentiation [78]. FK506-binding protein 52 (FKBP52) is a co-chaperone of PR and, in mice lacking FKBP52, epithelial differentiation fails and uterine receptivity is severely compromised due to decreased progesterone activity and excessive estrogen activity [79]. In addition, knockdown of the nuclear receptor co-activator steroid receptor coactivator2 (SRC2) in the uterus causes implantation failure due to the fact that PR relies on SRC2 to initiate the uterine decidual response [80,81]. Nuclear receptor coactivator-6 (NCOA6) disrupts endometrial receptivity as it degrades ERs through ubiquitination, which results in increased sensitivity of the uterus to estrogen and abnormal expression of progesterone-related genes [82]. Heart and neural crest derivatives-expressed protein 2 (Hand2) is a functional regulator of progesterone and a transcription factor expressed in the uterine stroma. It acts as a repressor of estrogen-mediated epithelial cell proliferation. Fibroblast growth factor (FGF) production maintains epithelial cell proliferation and stimulates the estrogen-induced pathway, and Hand2 expression inhibits this process, which would otherwise result in impaired endometrial receptivity [83]. Another transcription factor, chicken ovalbumin upstream promoter transcription factor-2 (COUP-TFII), is also present in the endometrial stroma and plays an important role in progesterone maintenance of endometrial receptivity. Progesterone first induces the Indian hedgehog (IHH) gene, which regulates COUP-TFII, and bone morphogenetic protein 2 (BMP2) is a downstream molecule induced by COUP-TFII, thereby promoting decidualization [84]. In addition, COUP-TFII causes progesterone inhibition of estrogen by decreasing ERα expression in epithelial cells during the uterine receptive period [85]. Studies on mouse models have also demonstrated that some PR-regulated genes, such as IHH, BMP2 and HAND2, are essential for implantation and decidualization [86]. These results suggest that normal uterine receptivity requires the complexity and accuracy of ER and PR signaling (see Figure 2).
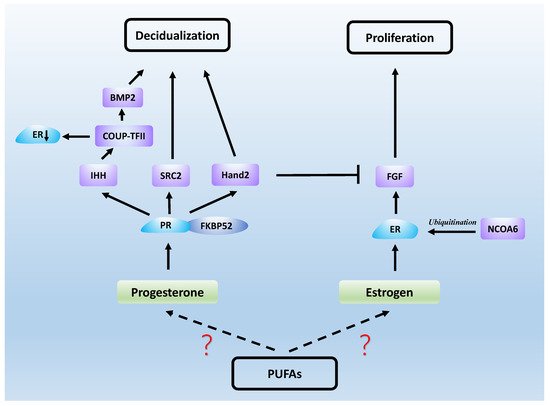
Figure 2. Steroid hormones work with a series of signaling molecules to generate uterine receptivity.
2.4. Androgen
Androgen is associated with PUFAs and also plays an important part in endometrial receptivity. Researchers observed that in overweight and obese men, intervention with DHArich fish oil was associated with an increase in testosterone concentrations [87]. Studies have shown that supplementation with n-3 PUFAs has no significant effect on androgen levels in women with polycystic ovarian syndrome(PCOS). However, some pre-term and long-term intervention studies have shown reduced levels of dehydroepiandrosterone (DHEA). Future studies need to be combined with double-blind placebo-controlled clinical trials and long-term follow-up [88]. Polyunsaturated fatty acid diets had better effects on hormone secretion and reproductive parameters in male buffalo compared to saturated fatty acid diets. The addition of n-3 PUFA levels to the ration increased the concentration of testosterone in the plasma and the scrotal circumference of male buffaloes and contributed to a shorter age at puberty [89].
The biological role of DHEA, particularly in fertility, is also controversial. A high androgen level impairs endometrial receptivity in women who have experienced recurrent miscarriages [90]. It was found that testosterone reduced the expression of pinopode and l-selectin ligands in the uterus during receptivity in rats, which may result in the failure of blastocyst implantation under conditions of high levels of this hormone [91]. Testosterone injection results in the loss of intrauterine tight junction complexity and the downregulation of intrauterine claudin-4 and occludin expression during the receptive phase, thus influencing embryo attachment and subsequent implantation [92]. Primary ESC was decidualized in vitro with progesterone and cAMP for 1–8 days with or without the androgen receptor (AR) antagonist flutamide. The addition of flutamide significantly altered the expression of endometrial receptivity indicators. Endometrial androgen biosynthesis during desiccation may play an important role in endometrial tolerance [93]. Another study found that DHEA enhanced in vitro decidualization response to human endometrial stromal fibroblast (hESF) in women of childbearing age. DHEA supplementation during the receptive phase enhances the endometrial function by enhancing the expression of the endometrial receptive marker secreted phosphoprotein 1 (SPP1). Meanwhile, flutamide (an androgen inhibitor) treatment can effectively improve decidualization, angiogenesis and uNK cell production due to hyperandrogenemia, further improving poor endometrial receptivity in PCOS patients [94]. Low concentrations of DHEA were found to increase the antioxidant capacity of metaphase ESCs in mice, and DHEA treatment also improved endometrial tolerance through AR [95]. In conclusion, testosterone is a novel negative regulator of endometrial receptivity.
This entry is adapted from the peer-reviewed paper 10.3390/biom12010036
This entry is offline, you can click here to edit this entry!
