Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Transient receptor potentialare cation channels made up of transmembrane proteins that function as transductors through changes in the membrane potential due to the intracellular concentrations of Ca2+. Q10 is the temperature coefficient of the rate of change when an organism increases its temperature by 10 °C.
- TRP
- ion channels
- mammals
- thermoregulation
- behavior
- welfare
1. Introduction
Thermoregulation plays a vital role in the survival of all endothermic organisms and altricial species, especially newborns [1][2][3][4]. Body temperature is a physiological and clinical parameter that provides relevant information on the individual’s state [5], while variations reflect valuable biomedical data [6]. Modulations of this parameter are closely related to the stability of numerous cardiovascular, respiratory, renal, endocrine, nervous, muscular, and cellular functions [7]. One example in humans is that the integrity of some cellular processes is altered within a specific range around the ideal temperature (37 °C) [8]. This characteristic of humans and non-human animals is known as the thermoneutrality zone (TNZ), which is defined as the environmental temperature range in which an organism does not need to activate metabolic and physiological pathways to dissipate or produce heat (heat loss, heat production) [9][10][11]. The precise TNZ range depends mainly on the species in question, physiological status (e.g., gestation), age, sex [12], body condition scores, and other factors that affect the thermoregulatory responses of the hypothalamus and preoptic nucleus [13][14].
Identifying between-species differences and determining the thermal comfort ranges of specific animal species is mediated by neurons in the central nervous system (CNS) and peripheral receptors [15][16]. Thermosensitive receptors exist in both prokaryotic and eukaryotic organisms [17]. The best-known central route of somatosensory cutaneous thermal signaling is the spinothalamic–cortical pathway, which originates in the activation of the system made up of thermoreceptors, thermosensors, and cutaneous effectors that carry signals of thermal stimuli to the dorsal root ganglion of the spinal cord. From there, the signals travel to the thalamus and, finally, to the primary somatosensory cortex, where body temperature is consciously perceived and integrated [18][19].
The sensory thermoreceptors in mammals are nerve endings with specialized ion channels that promote transitory modifications of membrane permeability, which depend on the external stimuli perceived [20]. Most of these are made up of transient receptor potentials that perform non-selective cation diffusion [21][22]; participate in the transduction of mechanical and chemical sensory stimuli and the maintenance of the membrane resting potential; and control calcium (Ca2+) and magnesium (Mg2+) levels in neurons and non-excitable or cancerous cells [23][24]. TRPs are expressed in almost all tissue cell types [25], excitable or non-excitable, and in all cell membranes except the nuclear and mitochondrial membranes. Most TRPs are located in the plasmatic membrane where they contribute significantly to numerous physiological processes and homeostatic functions, and participate in vasomotor control and muscular contraction [26].
2. Structure
TRP channels have a common primary structure similar to that of K+ channels. They consist of 4 subunits surrounding an ionic permeation [27]. Each subunit has six segments, or transmembrane domains [28], of which two (S5, S6) constitute the central pore, while the others (S1–S4) form a tetramer around it [29][28]. They contain long amino (N) and carboxyl (C) groups [30], which are located intracellularly. Since each subfamily of the TRP presents particularities in its soluble domains, a functional variability in each one can be distinguished [27] (Figure 1). TRP channels also have differential domains that have led them to be classified in distinct subfamilies whose morphological variations are based on comparisons of their amino acid sequences [31].
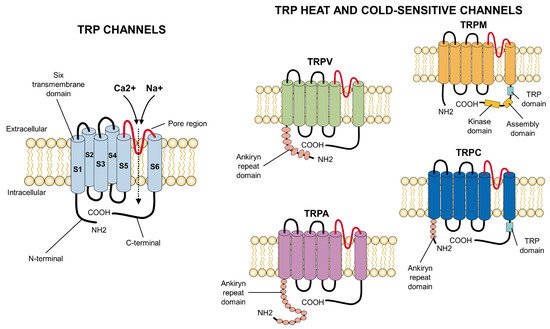
Figure 1. Primary structure of TRP ion channels. In general, all TRP channels are located in the cellular lipid bilayer and consist of six transmembrane subunits (S1–S6) with a pore region considered fundamental for the influx of cations, especially Ca2+ or Na+. The particularities which appear at the carboxyl- (C-terminal) or amino-terminus (N-terminal) (e.g., ankyrin repeats at the latter) differ among the families of TRP channels and confer specific properties to each receptor. COOH: carboxylic acid group; NH2: amino group.
Most of the channels responsible for modulating temperature are located in the somatic and visceral afferents. The cold receptors are small diameter, myelinated axons (A-delta) in primates, but C fibers in non-primate mammals [32]. The cold-sensitive channels include TRPC5, whose structure is similar to that of the other members of the TRPC family and consists of six transmembrane helices adhered to the ends of the N- and C-terminal domains in spiral form, a TRP domain, ankyrin repeats (around 3–4), and hydrophobic pores in the form of a loop [33]. The TRPM8 channel is also important in cold thermosensation. Its structure is similar to TRPV1 with six helicoidal segments, of which S5 and S6, which are added to the helix of pores, form the pore domain. Its cytoplasmatic region comprises the C-terminal and D-terminal domains. The latter includes four melastatin zones [34]. The TRPA1 channel is another cold sensor. It contains a transmembrane domain (composed of six helixes with a loop between S5 and S6), an N-terminal domain that presents 14–17 ankyrin repeats, and another cytosolic C-terminal; it lacks TRP domains [33].
The axons that transmit heat stimuli function through unmyelinated C fibers, which are predominant in somatic and visceral tissues [32]. These channels include TRPV1, which respond to temperatures identified as harmful. TRPV1 has a quadruple structure composed of a compact zone that occupies approximately 30% of the total volume and an open domain in a somewhat basket-shaped form that makes up the other 70% [35]. TRPV2 has structural similarities to TRPV1 since both have two constriction regions—wherein one is in the S6 helix (distal zone) and the other in the selectivity filter. However, TRPV1’s pore is narrower than TRPV2’s, which is of complete length and, hence, capable of partially accommodating hydrated cations and other large organic ions [33].
Other important channels for heat sensitivity are TRPV3 and TRPV4, which are activated by non-harmful temperatures. The architecture of TRPV3 is similar to the TRPV channels, as it has transmembrane ionic channel domains constituted by S1–S4, amphipathic helixes, and pores added to an intracellular skirt domain that connects to the ankyrin repeats and encapsulates a cytoplasmic cavity. However, differences in its transmembrane ionic channel and ankyrin repeat domains can be observed. In addition, it presents a looped C-terminal domain that has not been seen in the other members of its subfamily [36]. TRPV4’s structure differs from TRPV1 in the pore, which has only one constriction in the narrowest region (lower gate) and lacks the upper gate. In addition, its selectivity filter is wider, and the turn that its S1–S4 domains make is in a clockwise direction and, concerning the S4 helix, at 90° [37].
3. Heat-Sensitive TRP
3.1. Harmful Heat
3.1.1. TRPV1
This channel is responsible for perceiving harmful heat above 43 °C [19]. A non-selective channel that is permeable to cations, TRPV1 is activated by capsaicin, low pH (<6), temperature increases, and changes in membrane polarity [38]. It thus contrasts to TRPM8, which is activated by cold and menthol [17][39]. TRPV1’s activation range is 43–50 °C. Substances like ethanol also activate endogenous lipids (e.g., endocannabinoids, anandamides, and N-arachidonoyl dopamine), which are products of the lipoxygenase metabolism and topical analgesics. It is also associated with the recognition of harmful stimuli [17].
It is important to point out that diverse inflammatory mediators can reduce the temperature threshold for the activation of this thermoreceptor while increasing the magnitude of the responses of the ionic channel to produce inflammatory pain and heat hyperalgesia. For this reason, studies recommend that TRPV1 be a target for the development of analgesics [40]. In addition to its location in the sensory neurons of the dorsal root ganglia, TRPV1 is present, to a lower degree, in some cerebral structures, smooth muscle cells, adipocytes, the vascular system, and the gastrointestinal tract. Due to these circumstances, it has not yet been possible to precisely elucidate which sites TRPV1 contributes concretely to thermoregulation, for it also performs a function in regulating metabolism [40][41].
The observation that some TRPV1 knockout mice showed slight difficulties in detecting hot temperatures suggests that other mechanisms participate [42]. Reports describe the existence of a variant of the TRPV1 receptor in the sensory neurons of vampire bats that responds to a lower temperature threshold (~30 °C) and has been correlated with a delicate sensitivity that allows detection of warm-blooded prey [40][43].
3.1.2. TRPV2
TRPV2 is considered a receptor present in A-delta heat-sensitive fibers, whose activation is observed at harmful temperatures above 52 °C [19]. It is also associated with the perception of osmotic stress to mechanical stimuli and pharmacological compounds, like cannabidiol and tetrahydrocannabinol [17]. It is found in pulmonary tissue, the spleen, intestines, dorsal root ganglia, sensory ganglia, and the brain. TRPV2 is the principal receptor expressed in cerebral tissues (e.g., cerebellum, forebrain, and hippocampus). It has been suggested that in addition to its thermo-transductor functions, it may participate in the physiological processes in those cerebral structures [44]. Although TRPV2 presents 50% homology with TRPV1, it is not activated by capsaicin and is more permeable to Ca2+ than Na+ [45]. The role of this thermosensor has been studied in heat-sensitive nociceptors of rats, where administration of drugs like gadolinium—a TRPV2 antagonist—blocked the influx of cations, thus restraining the activation [44]. In contrast, the 2-aminoethoxydiphenyl borate agonist generates action potentials in the receptors in rats and mice, but not in humans [46]. Despite these findings, doubts persist concerning the participation of this receptor in thermoregulation because studies of TRPV2-deficient mice have not reported any aberrant thermosensitivity [47].
3.2. Harmless Heat
3.2.1. TRPV3
TRPV3 is a cation channel activated at warm temperatures around 34 °C [19], and ranging from 32–40 °C [48]. It belongs to the permeable channels group that are non-selective for Ca2+. It is expressed in the skin, keratinocytes, and oral and nasal mucosa, where its activation participates in other processes unrelated to thermosensitivity—such as hair growth, wound healing, itching, and pain perception [49]. Its presence in dermal tissues and the process of hair growth in mammals has led to studies of its participation in the processes of alopecia in hairless rodents. In those animals, mutations generate the absence of hairy structures due to an ionic alteration in the skin and observations of non-responsiveness to thermal stimuli and harmful chemicals [50]. Similarly, experimental results from Ferreira et al. [17] and Moqrich et al. [51] report that mice with genetic deficiencies of this receptor show altered responses to both harmless and harmful thermal stimuli. In contrast, Huang et al. [52] and Miyamoto et al. [53] mention that the TRPV3 and TRPV4 channels in mice do not play a fundamental role in heat perception or processes of heat hyperalgesia since animals deficient in this ionic channel did not manifest a deficit in their responses to heat stimuli. Variability in these results suggest that these phenotypes depend significantly on the genetic background of the mice tested [40].
3.2.2. TRPV4
This is a non-selective polymodal receptor similar to TRPV1 that is activated by moderately warm temperatures in the range of 27–34 °C [19]. In mammals, exposure to this temperature range, and the subsequent activation of TRPV4, generate the influx of Ca2+ and an action potential that helps them maintain thermal homeostasis concerning their environment or external stimuli [54]. Other functions of TRPV4 consist of acting as a sensor of hypotonicity [48] and as a mediator of such pathological processes as heat hyperalgesia and mechanical hyperalgesia. In both cases, excessive excitation due to direct damage, like spinal cord compression, leads to nitric oxide generation and a cascade that triggers sensitization processes like hyperalgesia and allodynia [55].
Other examples have been observed in species like mice when the TRPV4 gene is experimentally removed. This procedure revealed a deficit in perception of mechanical and chemical stimuli (tail pressure and the acetic acid test, respectively). However, those same subjects’ thermal responses to harmful heat (50 °C) were not altered, suggesting that one of TRPV4’s main roles is to perceive nociceptive mechanical stimuli rather than participating in thermal sensibility [56]. Like TRPV3, the fact that TRPV4 is heat-activated suggests that it participates in maintaining body temperature; however, no clear evidence exists to support this hypothesis [40].
3.2.3. TRPM2, TRPM4, and TRPM5
Since these three receptors require modulation by intermediaries (e.g., ADP-ribose, intracellular Ca2+, oxidative stress), and because they are mainly located in the brain, pancreas (TRPM2 specifically in β cells), and immune system cells, they tend to be excluded from the list of primary thermosensitive receptors [17][57]. Nonetheless, some studies have demonstrated their importance as thermal sensors involved in events like fever, where they intervene to prevent hyperthermia and its organic consequences, and their participation in hypothermia development since TRPM2 activation and inhibition modulate body temperature [58]. However, no thermosensory phenotype has been described in mice whose TRPM2 or TRPM4 receptor is inactivated. TRPM5 has been associated with the modulation of taste perception [59], but it remains unknown whether this characteristic acquires an important role in other homeostatic contexts [40].
4. Cold-Sensitive TRP
4.1. Harmful Cold
TRPA1
This receptor responds to a thermal threshold of 17 °C, which is considered harmful cold [19]. Increased intracellular Ca2+ concentrations mediate its activation due to the cold more than any direct action. Significantly, depolarization of TRPA1 is considered a fundamental element for inducing the cutaneous vasoconstriction responses that occur when individuals are exposed to low temperatures [60]. Some studies have found differences between species in which cold temperatures have activated TRPA1, like in rats and mice but not in humans or Rhesus monkeys [61]. Moreover, contrasting results have been reported within the same species (mice) since some authors indicate that this receptor contributes to the sensation of cold [62][63], but others rule out any such participation in detecting cold in vivo [64][65] or in mediating cold defense responses [66][67].
This receptor’s responses to harmful thermal (heat) and chemical stimuli have been studied in frogs and lizards, like the green anole (Anolis carolinensis). Saito et al. [68], for example, found that TRPA1 is activated under exposure to both conditions. A finding related to an evolutionary effect in which those species have preserved the TRPA1 channel for the detection of temperatures that significantly alter their homeostasis and, in this way, increase their probability of survival. Similarly, TRPA1 is associated with the transduction of nociceptive stimuli, like those linked to pro-inflammatory transmitters, such as the bradykinins [69].
4.2. Harmless Cold
4.2.1. TRPM8
This ion channel was the first one to be described as a thermoreceptor that specialized in the thermal sensation of cold. Activation of TRPM8 occurs under exposure to temperatures below 25 °C [19]. However, some authors mention that its activation begins at around 33 °C in the sensory neurons and that its signaling velocity increases proportionally to the temperature decrease [17][39][58]. This receptor is present in a subset of afferent neurons in the dorsal root ganglia that innervate the skin and sensory neurons of the trigeminal ganglion that innervate the head, eyes, and cornea [40]. There are also reports that TRPM8 is expressed in adipocytes, as mentioned with regards to TRPV1 [70][71].
Scientific evidence indicates that diverse, selective TRPM8 antagonists produce dose-dependent hypothermia in rats and mice. The topical application of menthol, a TRPM8 agonist, triggers hyperthermia and shivering-like muscle activity, vasoconstriction at the level of the skin of the tail, and heat-seeking behavior [72]. These findings concluded that there is a hypothermic effect dependent on this receptor’s activity [40].
4.2.2. TRPC5
TRPC5 is considered a cold-sensitive receptor that is activated at temperatures of 37–25 °C. It is present in the neurons of the dorsal ganglia and the dorsal lamina of the spinal cord [17]. Despite reports that describe the cold-induced gating of this receptor, studies of mice after ablation of the TRPC5 gene have concluded that those subjects do not present detectable defects related to cold sensitivity. Those findings call the role of this ion channel into question [40].
This entry is adapted from the peer-reviewed paper 10.3390/ani12010106
References
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–12.
- Nord, A.; Nilsson, J.F.; Sandell, M.I.; Nilsson, J.-Å. Patterns and dynamics of rest-phase hypothermia in wild and captive blue tits during winter. J. Comp. Physiol. B 2009, 179, 737–745.
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444.
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733.
- Mota-Rojas, D.; Gonçalves, C.; de Mira, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472.
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12.
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228.
- Picón-Jaimes, Y.A.; Orozco-Chinome, J.E.; Molina-Franky, J.; Franky-Rojas, M.P. Control central de la temperatura corporal y sus alteraciones: Fiebre, hipertermia e hipotermia. MedUNAB 2020, 23, 118–130.
- Kingma, B. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975–1985.
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited review: Influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 2016, 99, 2438–2452.
- Guo, Y.Y.; Hao, S.; Zhang, M.; Zhang, X.; Wang, D. Aquaporins, evaporative water loss and thermoregulation in heat-acclimated Mongolian gerbils (Meriones unguiculatus). J. Therm. Biol. 2020, 91, 102641.
- Mota-Rojas, D.; Miranda-Cortés, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato, L.; Hernández-Ávalos, I. Neurobiology and modulation of stress—Induced hyperthermia and fever in animals. Abanico Vet. 2021, 11, 1–17.
- Lizarralde, E.; Gutiérrez, A.; Martínez, M. Alteraciones de la termorregulación. Emergencias 2000, 12, 192–207.
- Vialard, F.; Olivier, M. Thermoneutrality and immunity: How does cold stress affect disease? Front. Immunol. 2020, 11, 588387.
- Mota-Rojas, D.; Wang, D.; Titto, C.G.; Gómez-Prado, J.; Carvajal-De la Fuente, V.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of fever and application of infrared thermography (IRT) in the detection of sick domestic animals: Recent advances. Animals 2021, 11, 2316.
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental applications and factors involved in validating thermal windows using infrared thermography to assess the health and thermostability of laboratory animals. Animals 2021, 11, 3448.
- Ferreira, G.; Raddatz, N.; Lorenzo, Y. Biophysical and molecular features of thermosensitive TRP channels involved in sensory transduction. In TRP Channels in Sensory Transduction; Madrid, R., Bacigalupo, J., Eds.; Springer: London, UK, 2015; pp. 1–39.
- González-Alonso, J. Human thermoregulation and the cardiovascular system. Exp. Physiol. 2012, 97, 340–346.
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion channels and thermosensitivity: TRP, TREK, or both? Int. J. Mol. Sci. 2019, 20, 2371.
- Oaklander, A.; Siegel, S. Cutaneous innervation: Form and function. J. Am. Acad. Dermatol. 2005, 53, 1027–1037.
- Alexander, S.; Mathie, A.; Peters, J. Ion channels. Br. J. Pharmacol. 2005, 144, 73–94.
- Wen, J.; Tingbei, B.O.; Zhao, Z.; Wang, D. Role of transient receptor potential vanilloid-1 in behavioral thermoregulation of the Mongolian gerbil Meriones unguiculatus. Integr. Zool. 2021, 0, 1–11.
- Clapham, D. TRP channels as cellular sensors. Nature 2003, 426, 517–524.
- Clapham, D.; Runnels, L.; Strübing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396.
- Wen, J.; Bo, T.; Zhang, X.; Wang, Z.; Wang, D. Thermo-TRPs and gut microbiota are involved in thermogenesis and energy metabolism during low temperature exposure of obese mice. J. Exp. Biol. 2020, 223, jeb218974.
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 1–11.
- Zhao, Y.; McVeigh, B.M.; Moiseenkova-Bell, V.Y. Structural pharmacology of TRP channels. J. Mol. Biol. 2021, 433, 166914.
- Liu, C.; Montell, C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25.
- Fernández-Carvajal, A.; Fernández-Ballester, G.; Devesa, I.; González-Ros, J.M.; Ferrer-Montiel, A. New strategies to develop novel pain therapies: Addressing thermoreceptors from different points of view. Pharmaceuticals 2011, 5, 16–48.
- Vangeel, L.; Voets, T. Transient receptor potential channels and calcium signaling. Cold Spring Harb. Perspect. Biol. 2019, 11, a035048.
- Montell, C. Drosophila TRP channels. Pflügers Arch.-Eur. J. Physiol. 2005, 451, 19–28.
- Jänig, W. Peripheral thermoreceptors in innocuous temperature detection. In Handbook of Clinical Neurology; Romavosky, A., Ed.; Elsevier: London, UK, 2018; Volume 156, pp. 47–56.
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. In Membrane Protein Complexes: Structure and Function; Harris, J., Boekema, E., Eds.; Springer: Singapore, 2018; pp. 141–165.
- Izquierdo, C.; Martín-Martínez, M.; Gómez-Monterrey, I.; González-Muñiz, R. TRPM8 channels: Advances in structural studies and pharmacological modulation. Int. J. Mol. Sci. 2021, 22, 8502.
- López-Romero, A.E.; Hernández-Araiza, I.; Torres-Quiroz, F.; Tovar-Y-Romo, L.B.; Islas, L.D.; Rosenbaum, T. TRP ion channels: Proteins with conformational flexibility. Channels 2019, 13, 207–226.
- Singh, A.K.; McGoldrick, L.L.; Sobolevsky, A.I. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 2018, 25, 805–813.
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A physio and pathophysiologically significant ion channel. Int. J. Mol. Sci. 2020, 21, 3837.
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824.
- Tan, C.H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016, 536, 460–463.
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temp. Multidiscip. Biomed. J. 2015, 2, 178–187.
- Riera, C.E.; Huising, M.O.; Follett, P.; Leblanc, M.; Halloran, J.; Van Andel, R.; De Magalhaes Filho, C.D.; Merkwirth, C.; Dillin, A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 2014, 157, 1023–1036.
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187.
- Gracheva, E.O.; Cordero-Morales, J.F.; González-Carcacía, J.A.; Ingolia, N.T.; Manno, C.; Aranguren, C.I.; Weissman, J.S.; Julius, D. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 2011, 476, 88–91.
- Leffler, A.; Linte, R.M.; Nau, C.; Reeh, P.; Babes, A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur. J. Neurosci. 2007, 26, 12–22.
- Clapham, D.E.; Montell, C.; Schultz, G.; Julius, D. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: Transient receptor potential channels. Pharmacol. Rev. 2003, 55, 591–596.
- Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Wang, Y.; Flores, C.M.; Qin, N. Activation properties of heterologously expressed mammalian TRPV2. J. Biol. Chem. 2007, 282, 15894–15902.
- Park, U.; Vastani, N.; Guan, Y.; Raja, S.N.; Koltzenburg, M.; Caterina, M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 2011, 31, 11425–11436.
- Desai, B.N.; Clapham, D.E. TRP channels and mice deficient in TRP channels. Pflügers Arch.-Eur. J. Physiol. 2005, 451, 11–18.
- Yang, P.; Zhu, M.X. TRPV3. Handbook of Experimental Pharmacology; Nilius, B., Flockerzi, V., Eds.; Springer: London, UK, 2014; pp. 273–291.
- Xiao, R.; Tian, J.; Tang, J.; Zhu, M.X. The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium 2008, 43, 334–343.
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472.
- Huang, S.M.; Li, X.; Yu, Y.; Wang, J.; Caterina, M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 2011, 7, 1744–8069.
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 1–12.
- Chung, M.-K.; Lee, H.; Caterina, M.J. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 2003, 278, 32037–32046.
- Ding, X.-L.; Wang, Y.-H.; Ning, L.-P.; Zhang, Y.; Ge, H.-Y.; Jiang, H.; Wang, R.; Yue, S.-W. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav. Brain Res. 2010, 208, 194–201.
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668.
- Vilar, B.; Tan, C.-H.; McNaughton, P.A. Heat detection by the TRPM2 ion channel. Nature 2020, 584, E5–E12.
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.D.C.; Heppenstall, P.; Wende, H.; Siemens, J.; De Castro Reis, F.; Heppenstall, P.; et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398.
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005, 438, 1022–1025.
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732.
- Chen, J.; Kang, D.; Xu, J.; Lake, M.; Hogan, J.O.; Sun, C.; Walter, K.; Yao, B.; Kim, D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 2013, 4, 2501.
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289.
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278.
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282.
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350.
- Chen, J.; Joshi, S.K.; Didomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172.
- de Oliveira, C.; Garami, A.; Lehto, S.G.; Pakai, E.; Tekus, V.; Pohoczky, K.; Youngblood, B.D.; Wang, W.; Kort, M.E.; Kym, P.R.; et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J. Neurosci. 2014, 34, 4445–4452.
- Saito, S.; Nakatsuka, K.; Takahashi, K.; Fukuta, N.; Imagawa, T.; Ohta, T.; Tominaga, M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J. Biol. Chem. 2012, 287, 30743–30754.
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857.
- Rossato, M.; Granzotto, M.; Macchi, V.; Porzionato, A.; Petrelli, L.; Calcagno, A.; Vencato, J.; De Stefani, D.; Silvestrin, V.; Rizzuto, R.; et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell. Endocrinol. 2014, 383, 137–146.
- Ma, S.; Yu, H.; Zhao, Z.; Luo, Z.; Chen, J.; Ni, Y.; Jin, R.; Ma, L.; Wang, P.; Zhu, Z.; et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 2012, 4, 88–96.
- Tajino, K.; Matsumura, K.; Kosada, K.; Shibakusa, T.; Inoue, K.; Fushiki, T.; Hosokawa, H.; Kobayashi, S. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2128–R2135.
This entry is offline, you can click here to edit this entry!
