In Portugal, publications with mechanochemical methods date back to 2009, with the report on mechanochemical strategies for the synthesis of metallopharmaceuticals. Since then, mechanochemical applications have grown in Portugal, spanning several fields, mainly crystal engineering and supramolecular chemistry, catalysis, and organic and inorganic chemistry.
1. Introduction
Green chemistry is based on the set of principles defined by Paul Anastas in 1988 [
1]. These twelve principles promote the reduction or elimination of use or generation of hazardous substances in the design, manufacture, and application of chemical products. Increasing concern about climate change and the establishment of the 17 Sustainable Development Goals by the United Nations demand an urgent paradigm shift both in scientific research and the industrial sector towards more sustainable and more environmentally friendly technologies [
2]. Nowadays, the world is facing numerous societal and economical challenges, from no net emissions of greenhouse gases to economic growth decoupled from resources usage. To face these challenges, it is crucial to reduce waste and create a more sustainable and “greener” industry. A growing interest in the design of low-environmental-impact processes has led to the search for alternative and innovative technologies capable of complying with recent guidelines, such as the European Green Deal [
3]. The European Green Deal provides an action plan to enhance the efficient use of resources by shifting to a clean, circular economy to restore biodiversity and reduce pollution. Two of the points that require action are investment in environmentally friendly technologies and support of industry innovation. Industry and academia have become more conscious of the importance of adopting more sustainable and environmentally friendly chemical processes, using safer solvents, and preventing waste [
4].
In recent years, mechanochemistry [
5] has grown as a promising alternative synthetic method in the various areas of inorganic, organic, and metal-organic chemistry. Mechanochemistry has shown to be capable of activating chemical transformations by mechanical forces, e.g., compression, shear, or friction in the solid phase, without the need of solvents, or for the use of trace amounts of solvents [
6,
7,
8].
Solid-state reactions allow for the exploration of new synthetic pathways, which can lead to better control over stereoselectivity, stoichiometric efficiency, atom economy, and formation of nanotechnological products. Moreover, different reaction processes have led to the investigation of and access to poorly soluble but cheaper reactants or new raw materials. Strong covalent bonds, such as carbon-carbon and carbon-heteroatom (C-N, C-O, C-halogen, C-S, C-Se, and C-Te), can be activated by solid-state grinding under mild conditions of temperature and reagents. Examples of organic covalent reactions performed by mechanochemistry include dehydrogenative coupling, oxidation, reduction, and organocatalytic reactions [
9,
10,
11,
12,
13,
14]. Mechanochemistry has also been showing promising advances in organometallic synthesis [
15].
Crystal engineering and supramolecular chemistry have also largely benefited from the use of mechanochemistry, as proven by the increasing number of publications reporting the synthesis of cocrystals, salts, metal-organic frameworks (MOFs), covalent-organic frameworks (COFs), and coordination polymers (CPs) by mechanochemical methods [
16,
17,
18,
19,
20]. The relevance of this technique is even more evident in the formation of pharmaceutical cocrystals, which can usually be better achieved by solid-state reactions rather than (re)crystallization from solution, as the milling processes are known to help to overcome the problem of the poor solubility of active pharmaceutical ingredients (API) [
21,
22].
Due to its characteristics, mechanochemistry is an emerging technique with potential to change the current dominance of wet chemical synthesis that is associated with sustainability; it was listed by IUPAC as one of the top 10 chemical innovations towards a sustainable future [
23,
24,
25].
In 2019, a COST Action focused on “mechanochemistry for sustainable industry” (CA18112, MechSustInd) [26] was initiated, aiming to promote fundamental and applied research in mechanochemistry and its implementation in European industry. This collaborative network is composed of partners from 33 COST-member countries, including Portugal and the team involved in this review, as well as 17 other Inclusiveness Target Countries and partners worldwide. Currently, MechSustInd is represented by a total of 77 academic and other institutions in Europe and around the world.
1.1. Mechanochemical Methods
Mechanochemical synthesis may be conducted under different conditions that often influence the outcome and the success of the reaction. The use of mechanochemical ball milling for the synthesis of the different types of compounds has been extensively reported over the last years. Amongst the different available mills, the most common are vibrational and planetary mills, with the latter being prone to scale-up, while the former is more suitable for small-scale synthesis. While in vibrational mills, jars swipe back and forth at a given frequency, in planetary ball mills, the jars rotate around a central axis and, at the same time, they spin around their own axis, creating centrifugal forces.
Stainless steel is the most common material used for the grinding jars and balls, but this may introduce metal contamination in the final bulk product. Available alternative materials are zirconia, tungsten carbide, polytetrafluoroethylene (Teflon), and poly(methyl)methacrylate (PMMA) (Figure 1).
Figure 1. Examples of screw and snap-closure stainless steel and PMMA grinding jars for vibrational mills.
From the point of view of designing the best strategy, it is also important to retain that the addition of catalytic substances (e.g., solvent) may control the reactivity. Besides grinding without the addition of any other compound other than the reagents (neat grinding—NG), the use of catalytic amounts of a solvent (liquid-assisted grinding—LAG) is quite often reported to accelerate or even enable reactions that would not occur by neat grinding. The definition of LAG is based on how mechanochemical reactivity is affected by the ratio of the liquid additive to the weight of reactants (η). An η value of 0 corresponds to neat grinding; if η > 10 μL/mg, then we have a solution reaction. In LAG, the η value lies in the range of ≈0–1 μL/mg. In this range, reactivity appears independent of reactant solubility, distinguishing LAG from slurry reactions (η > 1 μL/mg), in which low solubility can hinder reactivity [
27]. The addition of catalytic amounts of ionic salts (ion- and liquid-assisted grinding—ILAG) [
28,
29] and polymer (polymer-assisted grinding—POLAG) [
30,
31] is also known to play a role in some mechanochemical reactions.
Regardless of the success obtained with these techniques at lab scale, the scalability of such processes poses an issue for industrial applications. Even though the mechanochemical synthesis of drug-carrier composites at the 50 kg scale has been reported to be successful by ball milling, the typical values range from tens to hundreds of grams, and thus, mechanochemical synthesis by ball milling at large scale has not yet been fully proven [
32]. Twin screw extrusion (TSE) has been presented as an alternative mechanochemical approach for the continuous production of different compounds (such as cocrystals and MOFs) with high yield, purity, and crystallinity at kg·h
−1 rates with no added solvent or only minimal added solvent [
32].
1.2. Mechanochemistry in Portugal
The first publications regarding mechanochemistry being developed by Portuguese researchers date back to 2009 and 2010 and resulted from collaborations between the groups of Duarte, Braga, and Friščić at the Universities of Bologna and Cambridge, respectively. These first studies were based on the development of mechanochemical strategies for the synthesis of metallopharmaceuticals [
33] and metallodrugs [
34]. The ILAG synthesis of bismuth subsalicylate was an important step in the field, as it can be considered the first report on the success of this type of synthesis of a commercially available metallodrug, leading to shorter reaction times, milder conditions, higher yields, ease of the process, and a much more environment friendly process [
34].
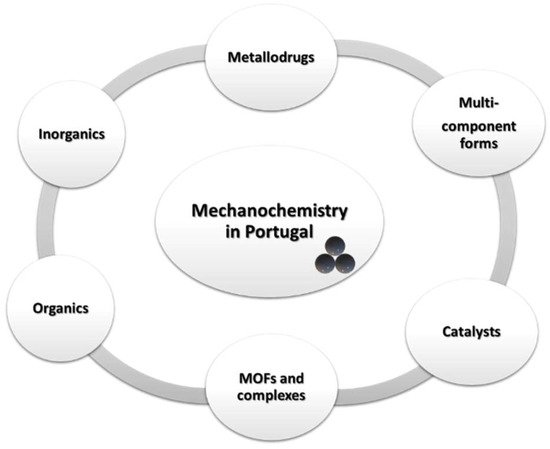
Almost simultaneously, the application of mechanochemistry to the synthesis of multicomponent crystal forms also sparked interest among Portuguese researchers (
Figure 2), especially regarding active pharmaceutical ingredients. This has been an area of growing interest in Portugal, with several research groups devoting their efforts towards the design and synthesis of solvates, salts, and cocrystals. More recently, the synthesis of pharmaceutical ionic liquids via these methods has also been reported as possible [
35].
Figure 2 expands the applications of mechanochemistry in Portugal.
Figure 2. Applications of mechanochemistry in Portugal.
2. Developments of Mechanochemistry in Portugal
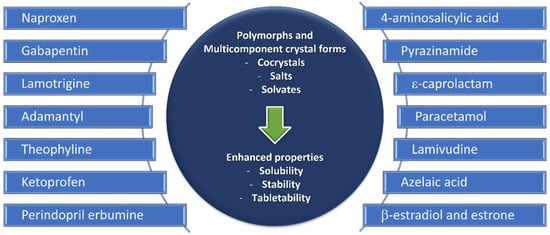
2.1. Mechanochemistry in the Synthesis of Polymorphs and Multicomponent Forms
Crystal Engineering comprises “the understanding of intermolecular interactions in the context of crystal packing and the utilization of such understanding in the design of new solids with desired physical and chemical properties”, as defined by Desiraju in 1989 [
38]. Molecular self-assembly is the driving force of crystal engineering, and supramolecular synthons defining crystalline packing can be either intermolecular interactions (e.g., hydrogen and halogen bonding) or coordination bonding. As such, crystal engineering comprises the study of polymorphs, hydrates/solvates, and different multicomponent crystal forms (salts, cocrystals, ionic cocrystals), according to the first approach (
Figure 3).
Figure 3. Polymorphs and multicomponent forms discussed in this review.
2.2 Mechanochemistry in the Synthesis of BioMOFs and Coordination Polymers
The advantages of mechanochemistry in the field of metallodrugs, metallopharmaceuticals, and bio-inspired metal-organic frameworks (BioMOFs) has also been unveiled by Portuguese research teams [76,77,78]. Within BioMOFs and metal complexes, antibiotics have been exploited as ligands seeking synergistic effects with different biocompatible metal sources [77]. This approach leads to antibiotic coordination frameworks (ACFs) and was motivated by the need to increase the efficiency of antibiotics that are becoming less effective due to antimicrobial resistance mechanisms.
2.3. Mechanochemistry in/for Catalysis
The application of mechanochemistry for the synthesis of catalysts has also been growing over the last years, mainly due to its ease of synthesis scalability, and sustainability, as well as the superior properties of the resulting materials [85]. Since 2014, several Portuguese groups have been involved in the mechanosynthesis of catalysts.
2.4. Mechanochemistry in the Synthesis of Organic and Inorganic Compounds
Mechanochemistry has been widely employed in the synthesis of inorganic and organic compounds, proving to be a powerful, low-cost, and green method with significant benefits in relation to conventional synthetic methodologies. Several examples of both organic and inorganic materials join the class of compounds successfully produced by mechanochemistry, in Portugal.
3. Final Remarks
The history of mechanochemistry in Portugal is still recent, dating back to 2009. However, a lot of significative work has already been disclosed in a wide range of fields. Examples using NG, LAG, and ILAG via manual grinding, vibrational ball milling, and hot-melt extrusion have been reported.
The development of new crystal forms of pharmaceutical compounds based in crystal engineering and supramolecular chemistry is undoubtedly the area where more work has been carried out. Several studies show the possibility of using mechanochemistry for the synthesis of polymorphs, solvates, salts, cocrystals, and ionic cocrystals towards improved physicochemical properties. BioMOFs and ACFs prepared by mechanochemistry in quantitative yield and short reaction times have been reported as alternative drug forms with enhanced solubility and/or efficiency.
Nevertheless, other non-pharmaceutical-related areas have also been explored, with significant results. Topics such as ionic liquids and liquid crystals have also benefited from the application of this synthetic procedure. The preparation of catalysts by mechanosynthesis was accomplished in very short reaction times and in quantitative yields, and their application in catalytic reactions showed effective results both in homo- and heterogeneous media. Moreover, syntheses of a variety of organic and inorganic derivatives were also performed by several research groups, proving mechanochemistry as a powerful and green methodology with significant benefits in relation to conventional solution-based methods.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27010241