Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Anthropology
The members of 68–78 kDa HSP subfamily (DnaK or HSPA, or HSP70s) are the major ATP-dependent chaperones of eukaryotes. The molecular structure of all HSP70s is rather conservative and exhibits the common domain organization with the N-terminal nucleotide-binding domain (NBD) and C-terminal substrate-binding domain (SBD) connected to each other by a flexible linker. In turn, the SBD is divided on a peptide-binding pocket and a bendable lid; these two subdomains allow the chaperone to transiently clasp a substrate protein molecule.
- HSP70 Subfamily
- Glucose-Regulated Protein 75
- Chaperone
1. Heat Shock Cognate Protein 70 (HSC70) and Inducible HSP70
The so-called heat shock cognate protein 70 (HSC70 or HSPA8) is the constitutively expressed form of HSP70s which is largely present in the cytoplasm. Under non-stressful conditions, cytosolic HSC70 catalyzes polypeptide chain folding in an ATP-dependent manner under maturation, transport or degradation of protein molecules. Mechanistically, the ATPase reaction with the binding and hydrolysis of ATP in the NBD followed by release of ADP and inorganic phosphate ensures the energy charge for HSP70-mediated (re)folding of protein substrates. In vivo, the interactions of HSC70 with ATP, ADP and protein substrates are regulated by (co-)chaperones and co-factors such as HSP40, HSP90, Hip, Hop, CHIP, BAG-1, BAG-3 and others that catalyze either folding and stabilization or degradation of various client proteins in the course of cyclic work of the ATP-consuming chaperone machine [1].
Additionally, the functions of HSC70 and its inducible forms can be regulated at the level of post-translational modifications of the chaperone molecule (phosphorylation, acetylation, methylation, ubiquitination, and others) [2]. One more method of the regulation of activities of HSP70 is its dimerization and oligomerization, since a dynamic pool of monomers, dimers, and oligomers of this HSP can exist in the cytosol [3]. All the above three ways of regulation of HSP70, namely at the level of (i) its interactions with co-chaperones and co-factors, (ii) its post-translational modifications and (iii) its oligomerization imply a possibility of multitarget inhibiting functions of HSP70 with different cell-permeable agents.
In the unstressed mammalian cell, the typical substrates of mammalian HSC70 are nascent polypeptides growing at ribosomes, clathrin and heat shock transcription factor 1 (HSF1). Similar activities, responses and subcellular localizations are also attributed to another member of the HSPA subfamily such as HSPA14 (HSP70L1). There are data that suggest the acquisition of radioresistance by human breast cancer cells is accompanied by the enhancement of HSPA14 expression [4]; however, a causal link between the intratumoral HSPA14 level and tumor radiation response has not been yet established. Together with HSP90, HSC70 binds to and inhibits HSF1, thus preventing the HSF1 activation under normal conditions. When denatured and aggregated proteins accumulate within the stressed cell, they recruit HSC70 and HSP90 from their complexes with HSF1 and the latter is activated to trigger the heat stress transcriptional response with subsequent expression of all stress-inducible HSP genes [5][6].
In the case of a proteotoxic stress, the inducible forms of HSP70 (often referred to as HSP72 and encoded by three very closely related paralogs: HSPA1A, HSPA1B, and HSPA1L) are expressed as a result of the stimulation of HSF1-mediated stress response that confers the improved survival/recovery and stress tolerance in HSP-enriched cells [1]. HSPA2 (HSP70-2) is known to be involved in spermatogenesis and also plays a role in breast cancer. One more stress-inducible form of HSP70 is HSPA6 whose cellular functions are less explored [7]. It is thought that the HSP70-conferred protection of stressed cells is mainly due to the ability of excess HSP70 (i) to attenuate the proteotoxicity by chaperoning stress-damaged cellular proteins and their aggregates [1] and (ii) to prevent the stress-induced cell death by blocking pathways that lead to apoptosis or necroptosis [8][9]. Besides its direct (inhibitory) interactions with the effectors of apoptosis [8][9], HSP70 can stabilize the inhibitor apoptosis proteins (IAPs) such as c-IAP1 and X-linked IAP (XIAP) [10]. In addition to its cytoprotective chaperoning of cell death regulators, HSP70 is able to modulate cellular signaling and protein degradation thereby maintaining the viability and fitness of stressed cells including the cancerous ones [8][9][11].
The inducible forms of HSP70 (HSPA1A, HSPA1B, and HSPA1L) are actively implicated in carcinogenesis as well as pathogenesis of cancer; many human malignancies exhibit the enhanced HSP70 expression which is commonly correlated with their aggressiveness and resistance to therapeutics [12][8][9]. A role of HSPA6 in cancer remains to be clarified. HSP70-enriched cancer cells are better adapted to the stressful conditions of the tumor microenvironment such as hypoxia, nutrient limitation, acidosis, etc. [13]. Notably, HSP70 can be exposed at the surface of malignant cells and also be secreted or incorporated into the extracellular vesicles (exosomes) [12][9][14]. A certain contribution of HSP70 to the development and pathogenesis of breast cancer is considered in the next sections.
2. Glucose-Regulated Protein 78 (GRP78)
The 78 kDa glucose-regulated protein (GRP78) (sometimes referred to as HSPA5 or binding immunoglobulin protein (BiP)) is an ATP-dependent chaperone and a member of the HSP70 subfamily; its molecule domain organization is similar to that of HSC70/HSP70 (see Figure 1). In vivo, GRP78 is implicated in the mechanisms of folding, degradation, transport and secretion of (glyco)proteins and also in the control over gene transcription, signaling pathways, Ca2+ homeostasis, autophagy and apoptosis [15].
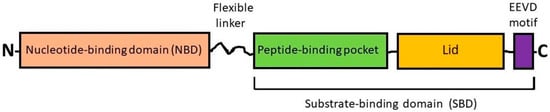
Figure 1. Simplified scheme showing the domain organization of HSP70s. The members of the HSP70 (HSPA) subfamily consist of two highly conserved functional domains such as the N-terminal nucleotide-binding domain (NBD) and the C-terminal substrate-binding domain (SBD) with a flexible linker between, and also have an EEVD motif at the C-terminus. The NBD contains the ATP/ADP pocket that binds ATP for the ATPase reaction. The SBD contains two subdomains: (i) a peptide-binding pocket that interacts with polypeptides as substrates and (ii) an α-helical subdomain from the C-terminal side that forms the so-called “lid”. The EEVD motif is involved in the interprotein interactions with co-chaperones and other HSPs [12][1].
GRP78 residing in the ER is known to be a cellular sensor of ER stress and master regulator of the so-called ‘unfolded protein response’ (UPR) that is triggered under hypoxia, hypoglycemia, Ca2+ ion imbalance, inhibition of protein glycosylation and other conditions inducing ER stress. In the unstressed cell, the constitutively expressed form of GRP78 within the ER lumen is in the complexes with the ER-specific stress signal transducers (SSTs) which are inactive being in that bound state. Under ER stress, GRP78 is recruited from those complexes by unfolded proteins and thus releases the three SSTs: protein kinase RNA-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 (IRE1); then the liberated SSTs are activated and trigger the UPR that leads to the stimulation of certain signaling pathways and expression of ER stress-responsive genes (reviewed in [13][15]). Among protein products of the GRP78-controlled UPR there are calreticulin, components of the ER-associated protein degradation (ERAD) machinery serving for the proteolysis and also inducible GRPs (GRP170, GRP94, GRP78, GRP75) which in an ATP-dependent fashion catalyze either the disaggregation/refolding or splitting of stress-damaged proteins within the ER and mitochondria. The intracellular level of GRP78 can be downregulated via the ubiquitination of GRP78 followed by its degradation, which is regulated by the acetylation/deacetylation of the chaperone molecule [16]. A minor part of GRP78 can be translocated to the cell surface or secreted outside the cell (including GRP78 being a component of exosomes), whereas a cytosolic isoform of GRP78 (GRP78va) can be produced via alternative splicing [13][15][17].
Intriguingly, GRP78 may sometimes promote cell death: if some GRP78-mediated responses to ER stress are cytoprotective (induction of chaperones, chaperone-mediated refolding or degradation of stress-damaged proteins, prevention of apoptosis), the other ones are proapoptotic (expression of CHOP and activation of caspases) [15]. Such ambivalent outcomes of the GRP78-controlled UPR appear to ensure the post-stress fulfilment of ‘quality control’ program: saving/recovery of stressed cells with reparable damages and apoptotic elimination of severely injured cells.
Both intracellular and extracellular GRP78 are involved in the mechanisms of malignant growth, cancer stemness maintenance, EMT, invasion and metastasis spread, and tumor resistance to therapeutics [15][17][18]. GRP78 and the GRP78-controlled UPR drive the metabolic reprogramming of cancer cells residing in the hypoxic regions of tumors [13][19]. Roles of GRP78 in breast cancer and its potential as of a targetable protein to combat this disease are reviewed in the next sections.
3. Glucose-Regulated Protein 75 (GRP75 or Mortalin)
GRP75, also named mortalin or HSPA9, or mitochondrial HSP75 (mtHSP75), or peptide-binding protein 74 (PBP74), is largely localized to the mitochondrial compartment where this ATP-dependent chaperone ensures the import of nuclear gene products. In cooperation with HSP60, GRP75 participates in the intramitochondrial protein folding and assembling of multimolecular protein complexes, thus maintaining the functional activity of mitochondria [17]. Moreover, mammalian GRP75 is thought to carry out the control of intracellular Ca2+ homeostasis, cell proliferation and senescence; by means of its direct interactions with p53 onco-suppressor protein, GRP75 regulates such p53-mediated events as the cell cycle arrest, DNA break repair and apoptosis or senescence following genotoxic stresses [18].
Importantly, the overexpression of GRP75 (including HSPA9B or mortalin-2) is found in many human malignancies which is causally correlated with their invasive growth, metastasis spread and resistance to chemotherapy and radiotherapy [13][18][20][21]. Tumoral GRP75 is known to contribute to the cancer stemness and EMT program that are associated with the aggressiveness and persistence of mammary gland carcinomas [18][22][23]. Additionally, GRP75 (mortalin) may present on the cancer cell surface, plays roles in cellular membrane trafficking and is a typical protein component of exosomes secreted by tumors of different origin including the breast tumors [24]. It is described in the next sections how GRP75 is implicated in the breast cancer pathogenesis and how this chaperone may be targeted to fight breast cancer.
This entry is adapted from the peer-reviewed paper 10.3390/cells10123446
References
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680.
- Nitika; Porter, C.M.; Truman, A.W.; Truttmann, M.C. Post-translational modifications of Hsp70 family proteins: Expanding the chaperone code. J. Biol. Chem. 2020, 295, 10689–10708.
- Takakuwa, J.E.; Nitika; Knighton, L.E.; Truman, A.W. Oligomerization of Hsp70: Current Perspectives on Regulation and Function. Front. Mol. Biosci. 2019, 6, 81.
- Miao, W.; Fan, M.; Huang, M.; Li, J.J.; Wang, Y. Targeted Profiling of Heat Shock Proteome in Radioresistant Breast Cancer Cells. Chem. Res. Toxicol. 2019, 32, 326–332.
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627.
- Pincus, D. Regulation of Hsf1 and the Heat Shock Response. Adv. Exp. Med. Biol. 2020, 1243, 41–50.
- Hageman, J.; van Waarde, M.A.W.H.; Zylicz, A.; Walerych, D.; Kampinga, H.H. The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem. J. 2011, 435, 127–142.
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256.
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587.
- Cesa, L.C.; Shao, H.; Srinivasan, S.R.; Tse, E.; Jain, C.; Zuiderweg, E.R.P.; Southworth, D.R.; Mapp, A.K.; Gestwicki, J.E. X-linked inhibitor of apoptosis protein (XIAP) is a client of heat shock protein 70 (Hsp70) and a biomarker of its inhibition. J. Biol. Chem. 2018, 293, 2370–2380.
- Fernández-Fernández, M.R.; Gragera, M.; Ochoa-Ibarrola, L.; Quintana-Gallardo, L.; Valpuesta, J.M. Hsp70—A master regulator in protein degradation. FEBS Lett. 2017, 591, 2648–2660.
- Vostakolaei, M.A.; Hatami-Baroogh, L.; Babaei, G.; Molavi, O.; Kordi, S.; Abdolalizadeh, J. Hsp70 in cancer: A double agent in the battle between survival and death. J. Cell. Physiol. 2021, 236, 3420–3444.
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 13, 1102.
- Elmallah, M.I.Y.; Cordonnier, M.; Vautrot, V.; Chanteloup, G.; Garrido, C.; Gobbo, J. Membrane-anchored heat-shock protein 70 (Hsp70) in cancer. Cancer Lett. 2020, 469, 134–141.
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23.
- Chang, Y.-W.; Chen, H.-A.; Tseng, C.-F.; Hong, C.-C.; Ma, J.-T.; Hung, M.C.; Wu, C.-H.; Huang, M.-T.; Su, J.-L. De-acetylation and degradation of HSPA5 is critical for E1A metastasis suppression in breast cancer cells. Oncotarget 2014, 5, 10558–10570.
- Conner, C.; Lager, T.W.; Guldner, I.H.; Wu, M.-Z.; Hishida, Y.; Hishida, T.; Ruiz, S.; Yamasaki, A.E.; Gilson, R.C.; Belmonte, J.C.I.; et al. Cell surface GRP78 promotes stemness in normal and neoplastic cells. Sci. Rep. 2020, 10, 3474.
- Kabakov, A.; Yakimova, A.; Matchuk, O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells 2020, 9, 892.
- Grkovic, S.; O’Reilly, V.C.; Han, S.; Hong, M.; Baxter, R.C.; Firth, S.M. IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene 2013, 32, 2412–2420.
- Havalová, H.; Ondrovičová, G.; Keresztesová, B.; Bauer, J.A.; Pevala, V.; Kutejová, E.; Kunová, N. Mitochondrial HSP70 Chaperone System—The Influence of Post-Translational Modifications and Involvement in Human Diseases. Int. J. Mol. Sci. 2021, 22, 8077.
- Srivastava, S.; Vishwanathan, V.; Birje, A.; Sinha, D.; D’Silva, P. Evolving paradigms on the interplay of mitochondrial Hsp70 chaperone system in cell survival and senescence. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 517–536.
- Zhang, R.; Meng, Z.; Wu, X.; Zhang, M.; Zhang, S.; Jin, T. Mortalin promotes breast cancer malignancy. Exp. Mol. Pathol. 2021, 118, 104593.
- Wei, B.; Cao, J.; Tian, J.-H.; Yu, C.-Y.; Huang, Q.; Yu, J.-J.; Ma, R.; Wang, J.; Xu, F.; Wang, L.-B. Mortalin maintains breast cancer stem cells stemness via activation of Wnt/GSK3β/β-catenin signaling pathway. Am. J. Cancer Res. 2021, 11, 2696–2716.
- Huang, M.-B.; Xia, M.; Gao, Z.; Zhou, H.; Liu, M.; Huang, S.; Zhen, R.; Wu, J.Y.; Roth, W.W.; Bond, V.C.; et al. Characterization of Exosomes in Plasma of Patients with Breast, Ovarian, Prostate, Hepatic, Gastric, Colon, and Pancreatic Cancers. J. Cancer Ther. 2019, 10, 382–399.
This entry is offline, you can click here to edit this entry!
