Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
|
Soil Science
Mercury (Hg) is an element with a high level of toxicity that poses a serious environmental threat. It is possible for Hg to enter the food chain and consequently affect human health, even at very low concentrations.
- PGPR
- mercury
1. Background
From a toxicological point of view, Hg is a toxic metal without any specific biological function. This element has a high potential for bioaccumulation and biomagnification due to the high solubility of Hg and methylmercury in fat and muscle tissue. An accumulation of Hg can result in pathologies of the central nervous system, such as Minamata’s Syndrome [3], as well as other health problems related to development, growth, and fertility [4].
On a global scale, anthropogenic emissions add approximately 2500 Mg of Hg to the atmosphere every year. In Europe, Lado et al. [5] developed a model of Hg distribution in the soil in 28 countries. According to their research, the average amount of Hg in European soil was around 40 μg kg−1. Soils in Northern Europe have a higher concentration of Hg than soils in the countries of Central and Southern Europe due to the fact that cold, wet weather promotes the accumulation of Hg in organic matter in soil [6]. Studies related to Hg distribution in soils adjacent to the Hg mines in the Almadén mining district reveal the presence of both high and extremely high levels of Hg (up to 8889 μg/g), while the concentration in sediment and water reaches levels of up to 16,000 μg/g and 11.2 μg/L, respectively [7].
In 2003, after more than 2000 years of activity, the mines closed due to a decrease in the demand for Hg, as well as changes in European regulations regarding this metal. With the aim of providing alternative uses for the Almadén soil, the scientific community has been working to develop strategies to mitigate the effects of Hg. Certain physicochemical methods have been developed that enable the elimination of this metal from soil, but the current trend is to use biological methods that are more environmentally friendly, based on biotechnological techniques such as bioremediation. This is the case with phyto-rhizoremediation, which involves the synergistic collaboration of plants and microorganisms for the purpose of remediating chemical compounds and pollutants from the environment [8]. An example of this activity is the use of plant growth-promoting rhizobacteria (PGPR) [9], which can be used in phyto-rhizoremediation aimed at the plant’s root in order to aid its physiological development, as well as direct activity aimed at the pollutant, while simultaneously increasing the effect of the plant itself on the pollutant.
Hg tolerance and Hg resistance of microorganisms can contribute to the reduction and/or elimination of the different types of Hg in contaminated environments, which has led to increased interest in the selection of bacterial strains with biotechnological potential, as well as their use in bioremediation [10].
The Bio-Mercury Remediation Suitability Index (BMRSI) has proven to be a useful tool for evaluating the Hg bioremediation potential of the bacterial strains, since it takes into account not only the Hg resistance capability of the bacteria, but also their combined PGPR capacity.
2. Current Insight on PGPR Strains
The selected strains were subjected to more extensive tests in order to identify the best candidates for further uses in phyto-rhizoremediation based on their PGPR capabilities in the presence of Hg.
For this purpose, only the data obtained in the Hg tests were analyzed due to the fact that the final objective of this study was to analyze the remediation capability in the presence of Hg, as well as the choice of the best strains for use in the bioremediation of plots contaminated with this heavy metal.
Figure 1 shows the trend of IAA production at different Hg concentrations over time (12 h, 24 h, and 48 h) in all the strains. Data measured at 12 h and 24 h were found to be significantly higher than those measured at 48 h (p < 0.05). By analyzing the mean values, it was found that during 12 h incubation period, the production of IAA was significantly higher at concentrations of 80 μg/mL and 100 μg/mL than at 120 μg/mL and 140 μg/mL of Hg (p < 0.05). However, in the incubation period of 24 h and 48 h, at concentrations of 80 μg/mL, 100 μg/mL, and 120 μg/mL, IAA production was significantly higher (p < 0.05 and p < 0.005, respectively), than at concentrations of 140 μg/mL Hg.
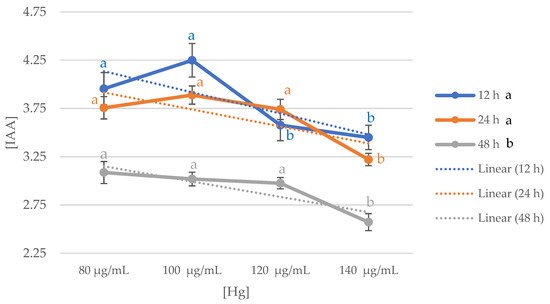
Figure 1. Average IAA production trend of the forty strains studied in relation to Hg concentrations at each of the measurement times. Letters a-b indicate the significance of p < 0.05, the black letters indicate significance differences among those grouped by hours; the blue letters indicate significance differences among the different concentrations of IAA measured at 12 h; orange letters indicate significance differences among different concentrations of IAA measured at 24 h; grey letters indicate significance differences among different concentrations of IAA measured at 48 h.
Therefore, it can be concluded that the average production value of the strains analyzed is obtained between 12 h and 24 h at concentrations between 80 μg/mL and 100 μg/mL. To select the range of data to be used later, values corresponding to the 12 h incubation period in mediums with a concentration of 100 μg/mL were used as a reference.
As shown in Table 1, only five strains (9, 48, 58, 122, and 173) exhibited ACCd activity. All of these showed activity up to concentrations of 100 μg/mL, and two of them (strains 9 and 58), up to 120 μg/mL of Hg.
Table 1. Strains ordered by BMRSI descending values with all factors integrated, IAA: IAA production; PO43−: solubilization of phosphates; ACCd: degradation of ACC via ACC deaminase; MBC: maximum bactericidal concentration. 0/1 indicates absence/presence. ND: not defined bacteria.
| Strain | Identification | IAA (μg/mL) | PO43− | ACCd | MBC (μg/mL) |
BMRSI |
|---|---|---|---|---|---|---|
| 9 | Bacillus toyonensis | 6.16 | 0 | 1 | 140 | 7.30 |
| 21 | Pseudomonas moraviensis | 7.06 | 0 | 0 | 140 | 7.20 |
| 98 | Pseudomonas baetica | 6.76 | 0 | 0 | 160 | 6.92 |
| 95 | Brevibacterium frigoritolerans | 6.40 | 0 | 0 | 140 | 6.54 |
| 37 | Pseudomonas fluorescens | 6.08 | 0 | 0 | 140 | 6.22 |
| 56 | Pseudomonas brassicacearum subsp. brassicacearum | 6.05 | 0 | 0 | 160 | 6.21 |
| 58 | Pseudomonas brassicacearum subsp. brassicacearum | 4.70 | 0 | 1 | 160 | 5.86 |
| 31 | Pseudomonas brassicacearum subsp. brassicacearum | 5.67 | 0 | 0 | 140 | 5.81 |
| 122 | Brevibacterium frigoritolerans | 4.37 | 0 | 1 | 160 | 5.53 |
| 50 | Bacillus toyonensis | 4.15 | 1 | 0 | 350 | 5.50 |
| 173 | Bacillus toyonensis | 3.93 | 0 | 1 | 180 | 5.11 |
| 48 | ND | 3.91 | 0 | 1 | 140 | 5.05 |
| 57 | Pseudomonas corrugata | 3.61 | 1 | 0 | 350 | 4.96 |
| 55 | Pseudomonas syringae pv. phaseolicola | 4.80 | 0 | 0 | 140 | 4.94 |
| 69-II | Pseudomonas sp. | 3.77 | 1 | 0 | 160 | 4.93 |
| 70 | Pseudomonas corrugata | 4.51 | 0 | 0 | 350 | 4.86 |
| 69-I | Pseudomonas syringae pv. phaseolicola | 4.67 | 0 | 0 | 160 | 4.83 |
| 43 | Bacillus toyonensis | 4.59 | 0 | 0 | 160 | 4.75 |
| 1 | Pseudomonas migulae | 4.59 | 0 | 0 | 140 | 4.73 |
| 23 | Pseudomonas moraviensis | 4.42 | 0 | 0 | 140 | 4.56 |
| 76 | ND | 4.10 | 0 | 0 | 140 | 4.24 |
| 204 | Brevibacterium frigoritolerans | 4.04 | 0 | 0 | 160 | 4.20 |
| 149 | Pseudomonas syringae pv. phaseolicola | 4.02 | 0 | 0 | 140 | 4.16 |
| 211 | Bacillus dendretensis | 3.85 | 0 | 0 | 200 | 4.05 |
| 114 | Pseudomonas syringae pv. phaseolicola | 3.80 | 0 | 0 | 140 | 3.94 |
| 75 | Pseudomonas syringae pv. phaseolicola | 3.74 | 0 | 0 | 160 | 3.90 |
| 79 | Pseudomonas syringae pv. phaseolicola | 3.66 | 0 | 0 | 140 | 3.80 |
| 74 | Xanthomonas oryzae pv. oryzae | 3.66 | 0 | 0 | 140 | 3.80 |
| 35 | Pseudomonas baetica | 3.64 | 0 | 0 | 140 | 3.78 |
| 20 | Pseudomonas fluorescens | 3.64 | 0 | 0 | 140 | 3.78 |
| 175 | ND | 3.50 | 0 | 0 | 140 | 3.64 |
| 130 | Pseudomonas corrugata | 3.47 | 0 | 0 | 140 | 3.61 |
| 18 | Bacillus toyonensis | 3.45 | 0 | 0 | 140 | 3.59 |
| 11 | Pseudomonas corrugata | 3.34 | 0 | 0 | 200 | 3.54 |
| 146 | Pseudomonas fluorescens | 3.20 | 0 | 0 | 180 | 3.38 |
| 10 | ND | 3.09 | 0 | 0 | 140 | 3.23 |
| 160 | Bacillus circulans | 3.09 | 0 | 0 | 140 | 3.23 |
| 211 | Bacillus dendretensis | 2.88 | 0 | 0 | 200 | 3.08 |
| 214 | Bacillus niacini | 2.82 | 0 | 0 | 200 | 3.02 |
| 80 | Pseudomonas syringae pv. phaseolicola | 2.85 | 0 | 0 | 140 | 2.99 |
Only three strains (50, 57, and 69-II) solubilize phosphates under the Hg conditions studied.
The production of siderophores was not included in Table 1 since no strain is safe to produce in the presence of Hg.
Regarding MBC, all strains resisted concentrations above 100 μg/mL. The minimum concentration resisted by the 40 strains was 140 μg/mL. A total of 55% of the strains tested resisted up to 140 μg/mL, but the other half had much higher resistance values. In the remaining 45%, we found nine strains that resisted up to 160 μg/mL, two up to 180 μg/mL, four up to 200 μg/mL, and three of them resisted up to 350 μg/mL.
Finally, after the analysis was carried out for each of the variables, the BMRSI was calculated using the data of the PGPR activity measured at 100 μg/mL in order to introduce the least possible variability and obtain uniform data from the sample.
Table 1 shows the integrated data of the PGPR and MBC activities of the 40 strains considered for evaluation using the BMRSI.
When the measurement is standardized at 100 μg/mL of Hg, it can be observed that the datum with greater weight in the calculation is the amount of IAA produced by each strain. Similarly, the production of siderophores for all the strains in the selected conditions is 0. Therefore, strains with high IAA production that exhibit other PGPR activity will have a higher BMRSI. Similarly, the taxonomic identification of the 40 selected strains can be observed in Table 1 [11].
As such, a percentage comparison was made of the number of bacteria that exhibit each of the PGPR activities at 0 μg/mL of Hg obtained by Robas et al. [11], compared to those obtained using the selection criterion of 100 μg/mL of the present study, as shown in Figure 2. As can be seen in Figure 2, a reduction in the PGPR capacity of the bacteria under study occurs when these activities are analyzed in the presence of Hg.
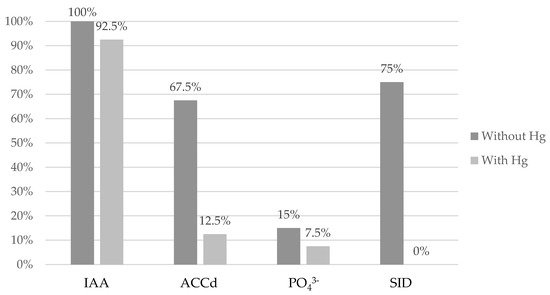
Figure 2. Percentage comparison of the data obtained by Robas et al. [11], contrasted with those obtained in the present study of the number of strains with PGPR activity at 0 μg/mL and 100 μg/mL of Hg. IAA: auxin producers; ACCd: ACC degraders; PO43−: phosphate solubilizers; and SID: siderophore producers.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph18189867
This entry is offline, you can click here to edit this entry!
