Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Two-dimensional amorphous nanomaterials (2D ANMs) are booming gradually and show promising application prospects in electrochemical fields for extended specific surface area, abundant active sites, tunable electron states, and faster ion transport capacity.
- two-dimensional amorphous nanomaterials
- single-layer
- electrochemical performance
1. Introduction
With the intensification of the global energy crisis, electrochemical energy storage and transformation has become one of the most concerned research hotspots in the world. Therefore, it is necessary to develop efficient, clean, and sustainable energy technologies, such as supercapacitor, battery, and electrocatalysis. As the core parts of these systems, electrode materials have experienced vigorous development and achieved multi-size, multi-dimensional, and multi-component precise regulation to adapt the diverse and complex energy storage and transformation processes [1][2][3][4].
Electrochemical performance is closely related to the structure of electrode materials. The two-dimensionalization of electrode materials can increase electrochemically active surface area (ECSA) and facilitate ion diffusion for enhanced electrochemical performance, which has drawn extensive attention [5][6][7][8]. Different from conventional material control strategies mainly concentrated upon composition, morphology, and dimension, crystal phase control demonstrates some superiority, especially for enhancing performance. Many materials have more than one phase, which is mainly determined by chemical bonds and thermodynamic parameters. By precisely controlling various structural parameters, it is possible to obtain non-thermodynamically stable phase structure with disordered atomic arrangement over a long range and only short-range order over a few atoms, that is amorphous structure. The materials with amorphous structure are isotropic, lack grain boundaries, and endowed with inherent abundant defects, which have come into people’s attention and worked as advanced electrode materials [9]. For instance, Lei et al. found amorphous titanium dioxide to be an efficient electrode material for sodium ion batteries with impressive charge storage capacity and cycle life [10]. Therefore, it is challenging but meaningful to combine the merits of two-dimensional and amorphous structures for developing well-performed, two-dimensional amorphous nanomaterials (2D ANMs). Compared with conventional materials, 2D ANMs generally exhibit distinctive features: i) ultra-high specific surface area and plentiful defects, which can provide more exposed active sites; ii) favorable diffusion paths and distances, which are conductive to the insertion/extraction of reactants and products; iii) strong in-plane covalent bond and lacking of grain boundary enhancing mechanical properties for extended volume or shape change; iv) flexible morphology and composition providing an additional degree of freedom for further modification; v) unprecedented electron state induced by confinement of electrons in 2D scale, which may facilitate electron transfer and electrode reactions.
2. Manipulation Strategy of 2D ANMs
2.1. Synthesis Methods
J. Kotakoski et al. firstly used electron irradiation to create 2D amorphous carbon material in 2011, which opened new possibilities for preparing 2D ANMs [11]. For some intrinsic bulk materials that are amorphous nanomaterials, the desired 2D ANMs can be obtained by exfoliation. For example, dimethylformamide (DMF) is an ideal solvent to exfoliate bulk MoS2 relative to other solvents due to its low surface tension (~40 mJ m−2) [12]. In view of this fact, 2D amorphous MoS3 nanosheets can be successfully obtained by the exfoliation of the bulk amorphous MoS3 material in DMF solvent under ultrasonic irradiation (Figure 1a) [13]. Other types of 2D ANMs have been obtained by many reliable methods including thermal decomposition [14], electrodeposition [15][16], template method [17][18][19], phase transformation [20][21], and element doping [22][23]. Synthesis of amorphous noble metal nanostructures is always a great challenge for their strong and isotropic nature of metallic bonds. In view of this, Li et al. proposed a simple method for preparing amorphous noble metal nanosheets by directly annealing metal acetylacetonate with alkali salt (Figure 1b) [24]. The synthesis temperature was between the melting point of metal acetylacetone and the melting point of alkali salt. When alkali salt was removed by deionized water, high yield amorphous noble metal nanomaterials can be successfully obtained, including monometal nanosheets, bimetal nanosheets, and trimetal nanosheets. Guo et al. utilized sacrificial template strategy to yield a library of ten distinct 2D ultrathin amorphous metal hydroxide nanosheets [18]. The key point of the synthesis is based on the balance between the etching rate of the Cu2O template and deposition rate of the metal hydroxide. As shown in Figure 1c, Cu2O was first employed as a sacrificial template to promote the 2D planar growth of metal hydroxides into a nanosheet structure. Then, S2O32− can react with Cu2O to produce OH− ions. Finally, after the concentrations of OH− ions increased to the precipitation threshold, metal ions could combine with OH− to form 2D amorphous sheet structure. In general, most of them are based on the classical 2D crystalline nanomaterials synthetic theory by introducing some mechanisms of inhibiting crystallization. The common inhibition factors involve shorting reaction time, reducing reaction temperature, destroying crystal structure, etc.
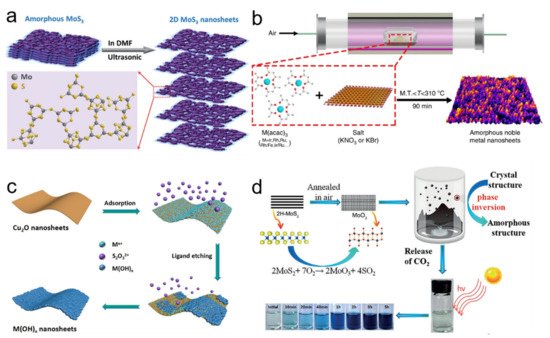
Figure 1. (a) Schematic illustration of exfoliation 2D amorphousMoS3 nanosheets and their chemical structure. Reprinted with permission from Ref. [13]. Copyright Royal Society of Chemistry, 2019. (b) Schematic illustration of the general synthetic process for amorphous noble metal nanosheets. Reprinted with permission from Ref. [24]. (c) The schematic illustration of the synthesis of amorphous metal hydroxide nanosheets. Reprinted with permission from Ref. [18]. Copyright Royal Society of Chemistry, 2019. (d) Schematic illustration of the formation mechanism for amorphous MoO3 nanosheets. Reprinted with permission from Ref. [21]. Copyright Wiley-VCH, 2017.
It needs to be clarified that some target amorphous products are difficult to prepare by one synthesis method and other methods should be involved. Xu et al. combined the oxidation of MoS2 and supercritical CO2 treatment strategy to prepare amorphous molybdenum oxide (MoO3) nanosheets [21]. As shown in Figure 1d, single-layer or few layers of crystalline MoS2 were firstly exfoliated. Then, oxygen atoms replaced sulfur atoms to destroy the regular atomic arrangement of MoS2 during the annealing process. Finally, the stable amorphous MoO3 was obtained by the adsorption of CO2.
2.2. Manipulation Modes
2D amorphous material has flexible structure and composition that allows dexterous manipulation. As mentioned above, various strategies have been proposed to manipulate 2D ANMs for enhanced electrochemical performance. We generalize and conceptualize these strategies to be two major categories of geometric configuration design and component interaction. According to our understanding, the geometric configuration design mainly includes spatial structure design at micro/nano scale and coordination environment design at atomic scale. The component interaction mainly includes elemental interaction and heterophase compositing. Here, the relevant enhanced effects and implementation approaches will be introduced.
2.2.1. Geometric Configuration Design
Spatial Structure Design
Spatial structure design at micro/nano scale can be deemed as the manipulation on the shape, size, packing form, and porous structure of 2D ANMs, which can be controlled by template design and reactive conditions [25]. Specifically, endowing 2D ANMs with porous structure should be an advisable way for enhanced electrochemical property. In electrocatalysis process, porous nanostructure can provide large surface area and abundant active sites, ensure effective penetration of electrolyte ions and escape of products, and alleviate stacking problem of nanosheets. As a typical case for creating pores on 2D ANMs, Guo group proposed a universal strategy combining confined method and ion exchange strategy to synthesize a series of 2D porous amorphous metal oxide nanosheets, such as Fe2O3, Cr2O3, ZrO2, SnO2, and Al2O3 [26]. The schematic illustration for synthesis of ultrathin amorphous metal oxide nanosheets was demonstrated in Figure 2a. Firstly, lamellar oleate was introduced as a host matrice to restrict the Cu2O template. Secondly, the target metal ion was replaced by Cu+ ions and introduced into 2D space through ion exchange strategy to form corresponding amorphous M(OH)x-oleate complex precursor. Finally, porous structure and disorder atom arrangement were achieved for metal oxide product by removing oleate and hydrone in heat treatment.
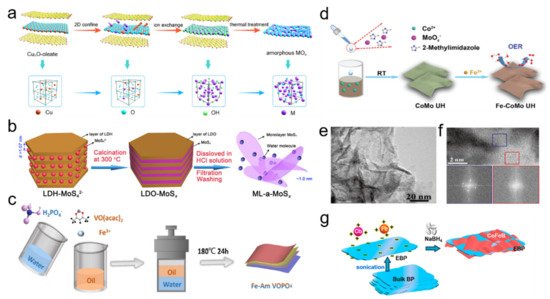
Figure 2. (a) Schematic illustration for synthesis of amorphous metal oxide ultrathin nanosheets. Reprinted with permission from Ref. [26]. Copyright American Chemical Society, 2020. (b) Schematic of the preparation process for amorphous MoSx monolayer nanosheets with abundant Mo defects. Reprinted with permission from Ref. [27]. Copyright Elsevier, 2020. (c) Schematic representation of the one-pot preparation processes of Fe-doped amorphous VOPO4. Reprinted with permission from Ref. [28]. Copyright Elsevier, 2021. (d) Schematic illustration of amorphous Fe-doped CoMo ultrathin hydroxide nanosheets. Reprinted with permission from Ref. [29]. Copyright Royal Society of Chemistry, 2021. (e) HRTEM image of CoV-Fe hydroxide nanosheets and (f) corresponding FFT patterns of selected regions marked by blue and red squares, respectively. Reprinted with permission from Ref. [30]. Copyright Wiley-VCH, 2020. (g) Schematic illustrations of the synthesis of EBP/CoFeB nanosheets. Reprinted with permission from Ref. [31]. Copyright American Chemical Society, 2021.
Coordination Environment Design
Tuning coordination environment at atomic scale can change the state of active sites, which afford improved electrochemical efficiency. Defect design is the most commonly used way and atomic-scale defects can be classified as anion vacancy, cation vacancy, associated vacancy, pits, distortions, and disorder [32]. Creating defects is generally deemed to be conductive to the mobility and adsorption of reactants and optimize reactive energy paths. In contrast to crystalline materials, precise design, and identification on defects are relatively difficult for amorphous ones with disorder atomic structure assembling massive and various defects, especially for 2D ANMs. Nonetheless, some efforts have been devoted to defect manipulation on 2D ANMs. Selective component removal or addition should be an effective way. Typically, Hou et al. developed amorphous MoSx monolayer nanosheets with abundant Mo defects using the space-confined strategy [27]. The synthesis details are shown in Figure 2b. The precursor of layered double hydroxide with MoS42− (LDH-MoS42−) was first synthesized via dispersing a layered double oxide (LDO) in an aqueous solution of (NH4)2MoS4. Afterwards, the obtained precursor was calcined in a N2 atmosphere to form amorphous MoSx monolayer nanosheets in the interlayer space of LDO. Finally, amorphous MoSx monolayer nanosheets were successfully obtained by washing in a nonoxidative HCl solution to dissolve LDO (the host layers). In this process, the generation of Mo defects can be adjusted by calcination temperature, which affects the S/Mo atomic ratio.
2.2.2. Component Interaction
Elemental Interaction
Doping or coupling other elements may be a feasible method to enhance the electrochemical performance of 2D ANMs due to the multielement synergy effect. Commonly adopted strategies are direct coupling and post-doping. Wei et al. fabricated Fe-doped amorphous VOPO4 in solvothermal environment by one-pot two-phase colloidal method (Figure 2c) [28]. The oil phase consisted of oleylamine (OM) and octadecene (ODE) dissolved with Fe and V precursor, which is mixed with water phase containing sodium dihydrogen phosphate, and then was sealed in an autoclave and heated to get the final product. Figure 2d demonstrated a typical post-doping way that crystalline CoMo ultrathin hydroxide was firstly constructed by coprecipitation reaction and then amorphous Fe-doped CoMo ultrathin hydroxide nanosheets was obtained by ion exchange process [29].
Heterophase Compositing
Compositing is a common means to combine a different phase with 2D ANMs. It can integrate advantages and realize optimized design on interfacial structure, holistic architecture and physical property, embody in enhanced conductivity, modulated electron structure and active sites, improved stability, etc. Recently, the introduction of crystal phase into the amorphous phase to form a crystalline/amorphous hybrid dual-phase structure has attracted much attention, due to the unique properties produced by the phase boundary. The flexible amorphous structure has abundant active centers, which can enhance the electrochemical activity, while the crystalline structure possesses a highly symmetrical nonflexible structure, which can enhance the stability of the material. Yan et al. prepared hybrid dual-phase materials by doping Fe in CoV hydroxide nanosheets composed of a large number of crystalline and amorphous domain mixtures (Figure 2e,f) [30].
The unique interfaces of the catalyst promote the exposure of the active center, adjust the local coordination environment and electronic structure, and reduce the thermodynamic barrier during the OER catalytic reaction. Carbon materials are desirable candidates to form compositing structure with 2D ANMs. Wen et al. reported an exfoliated black phosphorus/CoFeB nanosheet (EBP/CoFeB) implemented by three steps under a N2 atmosphere (Figure 2g) [31]. First, EBP was obtained from bulk phosphorus by liquid stripping; then metal ions (Co2+, Fe2+) were adsorbed on the surface of EBP through electrostatic interaction; finally, CoFeB nanosheets were grown on EBP through chemical reduction initiated by NaBH4. Both of them provide good demonstration on manipulating 2D ANMs by compositing way. Besides, many amorphous nanosheets deposited on various conductive substrates have been successfully synthesized to enhance the electrochemical performance, such as nickel foam [33][34], graphite [35][36], graphene [17], and TiO2 mesh [37].
This entry is adapted from the peer-reviewed paper 10.3390/nano11123246
References
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687.
- Augustyn, V.; Simonbc, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614.
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365.
- Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017, 2, 17059.
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098.
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, Z.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331.
- Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921.
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089.
- Li, Q.; Xu, Y.; Zheng, S.; Guo, X.; Xue, H.; Pang, H. Recent progress in some amorphous materials for supercapacitors. Small 2018, 14, 1800426–1800444.
- Zhou, M.; Xu, Y.; Wang, C.; Li, Q.; Xiang, J.; Liang, L.; Wu, M.; Zhao, H.; Lei, Y. Amorphous TiO2 inverse opal anode for high-rate sodium ion batteries. Nano Energy 2017, 31, 514–524.
- Kotakoski, J.; Krasheninnikov, A.V.; Kaiser, U.; Meyer, J.C. From point defects in graphene to two-dimensional amorphous carbon. Phys. Rev. Lett. 2011, 106, 105505.
- Gan, X.; Zhao, H.; Wong, K.; Lei, D.; Zhang, Y.; Quan, X. Covalent functionalization of MoS2 nanosheets synthesized by liquid phase exfoliation to construct electrochemical sensors for Cd (II). Talanta 2017, 182, 38–48.
- Fu, W.; Yang, S.; Yang, H.; Guo, B.; Huang, Z. 2D amorphous MoS3 nanosheets with porous network structures for scavenging toxic metal ions from synthetic acid mine drainage. J. Mater. Chem. A 2019, 7, 18799–18806.
- Selvaraj, A.R.; Muthusamy, A.; Inho-Cho; Kim, H.-J.; Senthil, K.; Prabakara, K. Ultrahigh surface area biomass derived 3D hierarchical porous carbon nanosheet electrodes for high energy density supercapacitors. Carbon 2021, 174, 463–474.
- Liu, W.; Liu, H.; Dang, L.; Zhang, H.; Wu, X.; Yang, B.; Li, Z.; Zhang, X.; Lei, L.; Jin, S. Amorphous cobalt-iron hydroxide nanosheet electrocatalyst for efficient electrochemical and photo-electrochemical oxygen evolution. Adv. Funct. Mater. 2017, 27, 1603904.
- Yu, L.; Zhou, H.; Sun, J.; Mishra, I.K.; Luo, D.; Yu, F.; Yu, Y.; Chen, S.; Ren, Z. Amorphous NiFe layered double hydroxide nanosheets decorated on 3D nickel phosphide nanoarrays: A hierarchical core-shell electrocatalyst for efficient oxygen evolution. J. Mater. Chem. A 2018, 6, 13619–13623.
- Zhao, H.; Zhu, Y.; Li, F.; Hao, R.; Wang, S.; Guo, L. A generalized strategy for the synthesis of large-size ultrathin two-dimensional metal oxide nanosheets. Angew. Chem. Int. Ed. 2017, 129, 8892–8896.
- Jia, B.; Hao, R.; Huang, Z.; Hu, P.; Li, L.; Zhang, Y.; Guo, L. Creating ultrathin amorphous metal hydroxide and oxide nanosheet libraries. J. Mater. Chem. A 2019, 7, 4383–4388.
- Zhao, H.; Yue, Y.; Zhang, Y.; Li, L.; Guo, L. Ternary artificial nacre reinforced by ultrathin amorphous alumina with exceptional mechanical properties. Adv. Mater. 2016, 28, 2037–2042.
- Jiang, Y.; Song, Y.; Pan, Z.; Meng, Y.; Jiang, L.; Wu, Z.; Yang, P.; Gu, Q.; Sun, D.; Hu, L. Rapid amorphization in metastable CoSeO3·H2O nanosheets for ultrafast lithiation kinetics. ACS Nano 2018, 12, 5011–5020.
- Liu, W.; Xu, Q.; Cui, W.; Zhu, C.; Qi, Y. CO2-assisted fabrication of two-dimensional amorphous molybdenum oxide nanosheets for enhanced plasmon resonances. Angew. Chem. Int. Ed. 2017, 56, 1600–1604.
- Liu, J.; Ji, Y.; Nai, J.; Niu, X.; Luo, Y.; Guo, L.; Yang, S. Ultrathin amorphous cobalt-vanadium hydr (oxy) oxide catalysts for the oxygen evolution reaction. Energy Environ. Sci. 2018, 11, 1736–1741.
- Lin, Z.; Du, C.; Yan, B.; Wang, C.; Yang, G. Two-dimensional amorphous NiO as a plasmonic photocatalyst for solar H2 evolution. Nat. Commun. 2018, 9, 1–11.
- Wu, G.; Zheng, X.; Cui, P.; Jiang, H.; Wan, X.; Qu, Y.; Chen, W.; Lin, Y.; Li, H.; Han, X.; et al. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019, 10, 1–11.
- Qin, C.; Fan, A.; Ren, D.; Luan, C.; Yang, J.; Liu, Y.; Zhang, X.; Dai, X.; Wang, M. Amorphous NiMS (M: Co, Fe or Mn) holey nanosheets derived from crystal phase transition for enhanced oxygen evolution in water splitting. Electrochim. Acta 2019, 323, 134756.
- Jia, B.; Yang, J.; Hao, R.; Li, L.; Guo, L. Confined synthesis of ultrathin amorphous metal-oxide nanosheets. ACS Mater. Lett. 2020, 2, 610–615.
- Wang, D.; Li, H.; Du, N.; Hou, W. Amorphous molybdenum sulfide monolayer nanosheets for highly efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2020, 398, 125685.
- Wei, Q.; Tan, X.; Zhang, J.; Yang, L.; Cao, L.; Dong, B. Fe doped amorphous single layered vanadyl phosphate nanosheets as highly efficient electrocatalyst for water oxidation. J. Colloid Interface Sci. 2021, 586, 505–513.
- Zeng, L.; Cao, B.; Wang, X.; Liu, H.; Shang, J.; Lang, J.; Cao, X.; Gu, H. Ultrathin amorphous iron-doped cobalt-molybdenum hydroxide nanosheets for advanced oxygen evolution reactions. Nanoscale 2021, 13, 3153–3160.
- Kuang, M.; Zhang, J.; Liu, D.; Tan, H.; Dinh, K.N.; Yang, L.; Ren, H.; Huang, W.; Fang, W.; Yao, J.; et al. Amorphous/crystalline heterostructured cobalt-vanadium-iron (oxy) hydroxides for highly efficient oxygen evolution reaction. Adv. Energy Mater. 2020, 10, 2002215.
- Chen, H.; Chen, J.; Ning, P.; Chen, X.; Liang, J.; Yao, X.; Chen, D.; Qin, L.; Huang, Y.; Wen, Z. 2D heterostructure of amorphous CoFeB coating black phosphorus nanosheets with optimal oxygen intermediate absorption for improved electrocatalytic water oxidation. ACS Nano 2021, 15, 12418–12428.
- Thomas, S.; Jung, H.; Kim, S.; Jun, B.; Lee, C.; Lee, S. Two-dimensional haeckelite h567: A promising high capacity and fast Li diffusion anode material for lithium-ion batteries. Carbon 2019, 148, 344–353.
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616.
- Yoon, S.; Yun, J.-Y.; Lim, J.-H.; Yoo, B. Enhanced electrocatalytic properties of electrodeposited amorphous cobalt-nickel hydroxide nanosheets on nickel foam by the formation of nickel nanocones for the oxygen evolution reaction. J. Alloys Compd. 2017, 693, 964–969.
- Ye, Y.-J.; Zhang, N.; Liu, X.-X. Amorphous NiFe(oxy)hydroxide nanosheet integrated partially exfoliated graphite foil for high efficiency oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 24208–24216.
- Gao, Y.Q.; Li, H.B.; Yang, G.W. Amorphous Co (OH)2 nanosheet electrocatalyst and the physical mechanism for its high activity and long-term cycle stability. J. Appl. Phys. 2016, 119, 034902.
- Wang, Z.; Ren, X.; Shi, X.; Asiri, A.M.; Wang, L.; Li, X.; Sun, X.; Zhang, Q.; Wang, H. A platinum oxide decorated amorphous cobalt oxide hydroxide nanosheet array towards alkaline hydrogen evolution. J. Mater. Chem. A 2018, 6, 3864–3868.
This entry is offline, you can click here to edit this entry!
