Surgical and locoregional treatments of peritoneal metastasis have gained increasing acceptance. Apart from systemic chemotherapy and surgical removal of the tumor, locoregional therapies such as CRS/HIPEC or PIPAC may improve tumor control.
1. CRS and HIPEC, a Locoregional Treatment Approach for Peritoneal Metastasis
A few decades ago, patients with PM from colorectal cancer (CRC) had a poor prognosis and seemed incurable [
43]. Over recent years, the management and treatment of PM has undergone major developments [
44]. The introduction of cytoreductive surgery (CRS), together with hyperthermic intraperitoneal chemotherapy (HIPEC), gave hope to patients with PM [
45,
46]. During CRS/HIPEC, to eradicate residual microscopic cancer cells, a heated solution with chemotherapeutic agents is applied perioperative directly into the peritoneum after macroscopically visible tumor nodules have been surgically removed. Historically, extensive debulking surgery started in around 1930 by J.V. Meigs for ovarian cancers, and the importance of cytoreductive surgery and, subsequently, of adjuvant chemotherapy became more evident over the next decades, not only for ovarian cancer, but also for pseudomyxoma peritonei [
47,
48,
49,
50]. With the increasing use of adjuvant chemotherapy, the idea of intraperitoneal application emerged to increase the therapeutic index [
51]. In the following years, several trials with CRS/HIPEC and optimization attempts with different chemotherapeutic agents, depending on histological origin, were conducted [
44]. To date, two main surgical intraperitoneal delivery techniques of hyperthermic intraperitoneal chemotherapy have been described: the open coliseum technique and closed administration [
52]. In the 1990s, Sugarbaker and his team proposed the peritoneal cancer index (PCI) as a standardized scoring system to quantify peritoneal tumor burden during an operation, and the completeness of cytoreduction score (CC score) to determine how radical the surgery was [
53]. The introduction of these scores is relevant for the selection of patients for CRS/HIPEC or to compare results among centers [
54]. The contemporary staging of PM is performed with the peritoneal cancer index (PCI), a staging system ranging from 0 to 39 points, which adequately describes the distribution and the size of PM in a patient [
55]. The comparison of PM with other metastatic sites is often problematic. Imaging modalities (e.g., computed tomography or magnetic resonance imaging) are more sensitive for metastasis in the lung or liver. Nevertheless, a review article on imaging diagnoses of PM from gastrointestinal and ovarian cancer showed an adequate pooled sensitivity and specificity of 80% and 90%, respectively, for PET-CT, and 92% and 85% for MRI. The authors claim that MRI could become the imaging modality of choice in staging PM, because MRI is already widely available [
56]. In a clinical trial (NCT03314649), the role of mutated DNA of cancer cells in the abdominal cavity of gastric cancer patients is assessed, with the goal of increasing sensitivity in the diagnosis of micrometastasis. Another interesting approach is the detection of peritoneal implants from CRC with near-infrared fluorescence imaging after the i.v. administration of indocyane green (ICG) (NCT02032485). Even though many new strategies in the diagnostic procedure are currently being investigated, current clinical practice still relies on the surgical staging of PM by either laparoscopy or laparotomy [
57].
Although the surgical concepts for cytoreduction are standardized, no standardization has been established thus far for HIPEC protocols with regard to temperature, treatment duration or the type of drug. Current HIPEC protocols for patients with colorectal PM therefore lack consistency and differ among countries and hospitals. For example, one proposed treatment protocol is the use of heated Oxaliplatin (Oxa), a third-generation platinum forming intra- and interstrand crosslinks in the DNA, for 30 min at 43 °C [58]. This protocol was also used in a prospective multicenter trial, PRODIGE-7. Patients (n = 265) with stage IV colorectal cancer with isolated peritoneal metastases and a PCI < 25 were randomly assigned to CRS or CRS with an oxaliplatin-based HIPEC for 30 min. No significant benefit regarding median overall survival could be shown by the addition of HIPEC (median OS 41.7 versus 41.2 months). Despite this result, the subgroup of patients with a PCI > 11 ≤ 15 demonstrated a significantly higher overall survival after CRS/HIPEC compared with CRS only [59]. The efficacy of HIPEC in general, with other drug combination, or at other concentrations cannot be answered by this study. Based on the results from both treatment groups, the main message of PRODIGE7 is that CRS, performed in expert centers, provides superior outcomes in patients with PM from CRC. The optimal regimen for HIPEC and the added benefit remains elusive [59].
Patient selection for CRS/HIPEC is key. Negative predictive factors after CRS/HIPEC are the extent of the disease (PCI), nodal stage, tumor biology, response systemic therapy or major complications after CRS/HIPEC. Some of these prognostic factors can be summarized by clinical scores (e.g., PDSS or BIOSCOPE). For example, BIOSCOPE includes the PCI, the nodal stage, tumor grading and the RAS/RAF mutational status, and helps to discriminate prognostic groups [
66]. In clinical practice, these scores are usually not exclusive, but may help in the clinical decision process. After CRS/HIPEC, a major complication (Clavien-Dindo classification IIIB or higher) rate of 8.3% to 24% has been published in recent series [
67,
68].
HIPEC can also be performed as an adjuvant treatment to prevent peritoneal spread. In their trial, Virzi et al. demonstrated the feasibility and safety of HIPEC in an adjuvant setting, even though 16% of patients experienced major complications [
69]. The COLOPEC trial could not prove any benefit in peritoneal-free survival after adjuvant HIPEC compared with the control arm [
70].
2. The Rapid Development of Systemic Chemotherapy
Since the use of 5FU, the field of medical oncology has evolved dramatically and new systemic regimens and targeted agents have significantly improved the survival of patients with advanced stages of CRC [
71]. Today, the choice of a systemic chemotherapy regimen depends decisively on the molecular pathological profile of the tumor. Frequently used systemic first-line regimens include combinations of 5FU or capecitabine with oxaliplatin or irinotecan [
18]. Triple therapy with 5FU, oxaliplatin and irinotecan (FOLFOXIR) is a very effective regimen and has demonstrated superior response rates [
72]. However, due to its side effects, it is usually given to patients in a good health condition [
73]. Targeted therapies, e.g., cetuximab, an EGFR-antibody, or bevacizumab, a humanized IgG monoclonal antibody targeting VEGF-A, are often added, depending on the molecular profile of the tumor. Multiple trials, including the phase III CRYSTAL and PRIME study, showed that adding an anti-EGFR to FOLFIRI and FOLFOX4, respectively, is beneficial for patients with RAS wild-type tumors [
20]. Overall, median overall survival has dramatically improved to nearly 30 months in the context of aggressive chemotherapy, which often enables secondary surgery of metastasis [
72] (
Table 1). The majority of patients included in these trials, however, have hematogenous metastasis, which has a significantly better prognosis than peritoneal metastasis [
4,
5]. Nevertheless, response to neoadjuvant systemic treatment is also a prognostic factor in patients with PM [
74]. Thus, progress in this field is likely to enable more aggressive surgery in the future.
Table 1. Current data on the treatment of PM from CRC by either systemic therapy alone or in combination with locoregional treatment. It is critical to highlight that the amount of disease in the peritoneum or the chemotherapy regimen differed among the studies. (amount of disease: +++ extensive load of PM, ++ moderate load, + limited load, NA: not available).
| PM—CRC Outcome after Different Treatment Approaches |
| |
mOS (Months) |
|
|
| |
CRS/HIPEC |
Systemic Chemotherapy |
PIPAC |
Amount of Disease |
Used Drug |
| Vervaal V. et al., 2003 [60] |
22.2 |
12.6 |
- |
+++ |
5FU |
| Elias D. et al., 2009 [58] |
67.7 |
23.9 |
- |
++ |
FOLFOX/FOLFIRI |
| Franko J. et al., 2016 [5] |
16.3 |
- |
- |
NA |
FOLFOX/FOLFIRI +/−ab |
| Cremolini Ch. et al., 2020 [75] |
- |
28.9 |
- |
NA |
FOLFOXIRI |
| Quenet F. et al., 2021 [59] |
41 |
- |
- |
++ |
FOLFOX/FOLFIRI |
| Breuer E. et al., 2021 [4] |
51 |
- |
- |
++ |
FOLFOX/FOLFIRI |
| Demtröder C. et al., 2015 [76] |
- |
- |
15.7 |
+++ |
FOLFOX/FOLFIRI +/−ab |
| Goére D. et al., 2020 [77] |
mOS not reached during 50.8 months of follow-up |
- |
|
+ |
FOLFOX/XELOX |
3. Novel Concepts and New Treatment Strategies for the Treatment of PM
Apart from using HIPEC as a complementary treatment, directly after cytoreductive surgery, some novel concepts have been introduced. Many of them highlight the palliative aspect of PM. A good example is pressurized intraperitoneal aerosol chemotherapy (PIPAC), where low-dose chemotherapy is applied intraperitoneally during laparoscopy as an aerosol at a pressure of 12 mmHg, usually every four weeks. This method is currently used in patients with non-resectable disease and is well tolerated [
78]. The rationale for PIPAC is a more homogeneous intraperitoneal distribution of chemotherapy in a closed space by applying it as a pressurized aerosol, leading to increased chemotherapy concentrations within the tissue compared with HIPEC [
77,
78]. PIPAC also offers a possibility to repeat the treatment. A further development of PIPAC is electrostatic PIPAC (ePIPAC). Here, a phase II study (NCT03246321) has completed patient recruitment using ePIPAC with oxaliplatin as a palliative monotherapy for patients with isolated and unresectable PM-CRC.
There are multiple challenges in the diagnosis and treatment of peritoneal metastasis. A major issue is the detection of minimal disease during surgery, where scars from previous procedures are impossible to differentiate from metastatic lesions. Techniques such as fluorescent-guided surgery could amplify detection sensitivity, making resections more precise. Several therapeutic targets have being tested; among them, anti-CEA antibodies have shown encouraging clinical results [
79]. Another field of research is the delivery of drugs into the peritoneal cavity. Here, apart from HIPEC or PIPAC, several technologies have been investigated that may improve the exposure of PM lesions to cytostatic drugs. For example, nanoparticles could provide several advantages, e.g., prolonged drug retention time, controlled drug release, and serve as a versatile vector for different molecules [
80]. The combined used of microspheres and hyaluronic acid hydrogel showed good results, enhanced drug solubility, prolonged drug release and sustained biocompatibility [
81]. Another innovative concept is NIPS, where a intraperitoneal catheter is placed into the pelvic cavity to administer neoadjuvant intraperitoneal and systemic chemotherapy. In patients with peritoneal metastasis from gastric cancer, NIPS resulted in a lower cancer-cell-positive ascites rate and in a higher R0 resection rate [
82].
Given the increasing knowledge on anti-tumor immunity in the peritoneum, immune checkpoint inhibitors may play a role, not only in micro-satellite instability-high (MSI-H), or mismatch repair-deficient (MMRd) metastatic colorectal or ovarian cancer [
83,
84,
85]. Although the immune cell landscape in PM lesions is not yet established, locoregional treatment may influence immunity in PM lesions. For example, anthracyclines—such as doxycycline—induce immunogenic cell death (ICD) [
86], which leads to the release of DAMPs from dying cancer cells. DAMPs in combination with antigen release can result in DC maturation and antigen presentation to CD8+ T cells [
86,
87]. After recognizing a specific antigen, T cells clonally expand and can fight surviving cancer cells. Moreover, cisplatin (also used for HIPEC) sensitized spontaneous lung cancer in a mouse model to induce a checkpoint blockade via the induction of ICD. This immunogenic effect is mediated not only by chemotherapy, but also radiotherapy; a single carefully selected dose is able to mediate similar effects. Sharma et al. described an enhanced expression of cancer testis antigens and MHC-I expression after the application of 20 Gy radiotherapy in vitro [
88]. Taken together, locoregional therapy may induce ICD to activate the immune system. In the context of HIPEC, the addition of hyperthermia might have an additional effect through the induction of heat shock proteins. In mice, an Hsp-90-mediated anticancer immune response was observed after the in vitro HIPEC treatment of murine cancer cells [
89]. An interesting example for a novel treatment approach for PM-CRC was provided by the phase 1 ImmunoPeCa trial. The immunotoxin MOC31PE was intraperitoneally administered one day after CRS and HIPEC, with the intention of killing EpCAM-positive cancer cells and preventing recurrence [
90].
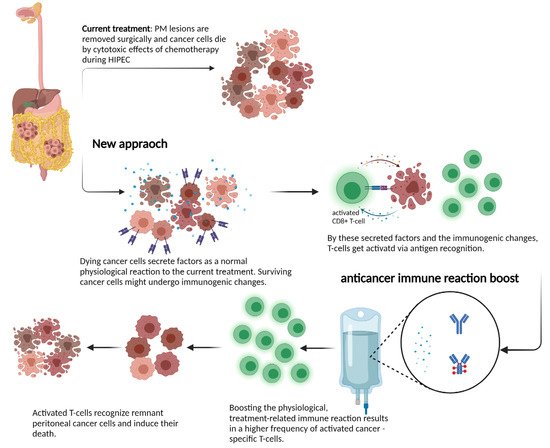
Despite the recent progress made, a deeper insight and molecular understanding of effects during locoregional treatment on the human host physiology and the immune system is required. Investigating tumor samples from PM patients and further assessments of cytokine profiles before and after treatment could provide critical knowledge about these processes. If surgery and locoregional treatment can induce anti-tumor immunity, these patients might profit from an additional treatment to boost the immune reaction. The rationale behind such a novel treatment approach is shown in Figure 1.
Figure 1. Schematic view of mechanisms of locoregional treatment in the peritoneum: the current interpretation of how HIPEC or PIPAC act on tumor cells is to induce cell death via direct cytotoxicity. Growing evidence on immunogenic cell death suggests that the activation of a patient’s immune system might mediate better long-term disease control through the induction of T cells. This process might be boosted in next-generation treatment approaches and induce a profound and sustained immune reaction against metastatic lesions. Created with BioRender.com.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14010060