Perennial herbaceous Fallopia is native to East Asia, and was introduced to Europe and North America in the 19th century as an ornamental plant. Fallopia has been spreading quickly and has naturalized in many countries. It is listed in the world’s 100 worst alien species. Fallopia often forms dense monospecies stands through the interruption of the regeneration process of indigenous plant species. Allelopathy of Japanese knotweed (Fallopia japonica), giant knotweed (Fallopia sachalinensis), and Bohemian knotweed (Fallopia x bohemica) has been reported to play an essential role in its invasion. The exudate from their roots and/or rhizomes, and their plant residues inhibited the germination and growth of some other plant species. These knotweeds, which are non-mycorrhizal plants, also suppressed the abundance and species richness of arbuscular mycorrhizal fungi (AMF) in the rhizosphere soil. Such suppression was critical for most territorial plants to form the mutualism with AMF, which enhances the nutrient and water uptake, and the tolerance against pathogens and stress conditions.
- allelochemical
- decomposition
- exudation
- invasive plant
- mycorrhizal colonization
- monospecies stand
- phytotoxicity
1. Introduction
2. Allelopathy of Knotweeds
| Source | Knotweed | Target Plant Species | Inhibition | Reference | |
|---|---|---|---|---|---|
| Root, rhizome | |||||
| Exudation | Japanese knotweed | Salix viminalis, Salix atrocinerea, Populus nigra | Growth | [50] | |
| Giant knotweed | Lactuca sativa | Growth | [51] | ||
| Rhizome extract | Japanese knotweed Giant knotweed Bohemian knotweed |
Leucosinapis alba | Growth | [52] | |
| Japanese knotweed Bohemian knotweed |
Raphanus sativus | Growth Germination |
[53][54] | ||
| Japanese knotweed | Atrichum angustatum Thuidium delicatulum |
Biomass | [55] | ||
| Above-ground part | |||||
| Leaf residue | Japanese knotweed Giant knotweed Bohemian knotweed |
Leucosinapis alba Brassica napa |
Germination | [56] | |
| Leaf extract | Japanese knotweed Giant knotweed Bohemian knotweed |
Urtica dioica Calamagrostis epigejos Lepidium sativum |
Germination | [57] | |
| Soaking water | Japanese knotweed | Triticum aestivum Sinapis arvensis |
Germination | [58] | |
| Extract (whole part) | Japanese knotweed Giant knotweed |
Brassica napa, Avena sativa Helianthus annuus |
Growth | [59] |
2.1. Exudation
2.2. Plant Residues
3. Invasion and Allelopathy of Knotweeds
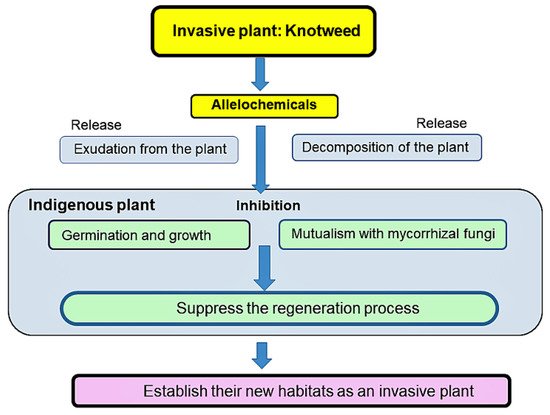
This entry is adapted from the peer-reviewed paper 10.3390/plants11010003
References
- Bailey, J.P.; Bímová, K.; Mandák, B. The potential role of polyploidy and hybridisation in the further evolution of the highly invasive Fallopia taxa in Europe. Ecol. Res. 2007, 22, 920–928.
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual spread versus sexual reproduction and evolution in Japanese knotweed s.l. sets the stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203.
- Sukopp, H.; Starfinger, U. Reynoutria Sachalinensis in Europe and in the Far East: A Comparison of the Species Ecology in its Native and Adventive Distribution Range. In Plant Invasions—General Aspects and Special Problems; Pyšek, P., Prach, K., Rejmánek, M., Wade, M., Eds.; SPB Academic: Amsterdam, The Netherlands, 1995; pp. 151–159.
- Adachi, N.; Terashima, I.; Takahaski, M. Central die-back of monoclonal stands of Reynoutria japonica in an early stage of primary succession on Mount Fuji. Ann. Bot. 1996, 77, 477–486.
- Tiébré, M.S.; Vanderhoeven, S.; Saad, L.; Mahy, G. Hybridization and sexual reproduction in the invasive alien Fallopia (Polygonaceae) complex in Belgium. Ann. Bot. 2007, 99, 193–203.
- Rahmonov, O.; Czylok, A.; Orczewska, A.; Majgier, L.; Parusel, T. Chemical composition of the leaves of Reynoutria japonica Houtt. and soil features in polluted areas. Cent. Eur. J. Biol. 2014, 9, 320–330.
- Mandák, B.; Pyšek, P.; Lysák, M.; Suda, J.; Krahulcová, A.; Bímová, K. Variation in DNA-ploidy levels of Reynoutria taxa in the Czech Republic. Ann. Bot. 2003, 92, 265–272.
- Mandák, B.; Pyšek, P.; Bímová, K. History of the invasion and distribution of Reynoutria taxa in the Czech Republic: A hybrid spreading faster than its parents. Preslia 2004, 76, 15–64.
- Tiébré, M.S.; Saad, L.; Mahy, G. Landscape dynamics and habitat selection by the alien invasive Fallopia (Polygonaceae) in Belgium. Biodivers. Conserv. 2008, 17, 2357–2370.
- Lamberti-Raverot, B.; Piola, F.; Thiébaut, M.; Guillard, L.; Vallier, F.; Puijalon, S. Water dispersal of the invasive complex Fallopia: The role of achene morphology. Flora 2017, 234, 150–157.
- Brock, J.H.; Child, L.E.; Waal, L.C.; Wade, M. The Invasive Nature of Fallopia Japonica is Enhanced by Vegetative Regeneration from Stem Tissues. In Plant Invasions—General Aspects and Special Problems; Pyšek, P., Prach, K., Rejmánek, M., Wade, M., Eds.; SPB Academic: Amsterdam, The Netherlands, 1995; pp. 131–139.
- Drazan, D.; Smith, A.G.; Anderson, N.O.; Becker, R.; Clark, M. History of knotweed (Fallopia spp.) invasiveness. Weed Sci. 2021, 69, 617–623.
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745.
- Bailey, J.P.; Conolly, A.P. Prize-winners to pariahs—A history of Japanese knotweed s.l. (Polygonaceae) in the British Isles. Watsonia 2000, 23, 93–110.
- Bailey, J.; Wisskirchen, R. The distribution and origins of Fallopia × bohemica (Polygonaceae) in Europe. Nord. J. Bot. 2006, 24, 173–199.
- Bailey, J.P. Japanese Knotweed s.l. at Home and Abroad. In Plant Invasions: Ecological Threats and Management Solutions; Child, L., Brock, J., Brundu, G., Prach, K., Pyšek, P., Wade, P., Williamson, M., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 183–196.
- Sołtysiak, J.; Brej, T. Characteristics that make the Fallopia genus (Polygonaceae) highly invasive. Ecol. Quest. 2012, 16, 23–27.
- Barney, J.N. North American history of two invasive plant species: Phytogeographic distribution, dispersal vectors, and multiple introductions. Biol. Invasions 2006, 8, 703–717.
- USDA PLANTS Database Profile: URl: Polygonum Cuspidatum, Polygonum Sachalinense. Available online: https://plants.sc.egov.usda.gov/home/plantProfile?symbol=POCU6 (accessed on 27 October 2021).
- New York Invasive Species Information, Profile: Japanese Knotweed. Available online: http://nyis.info/invasive_species/japanese-knotweed/ (accessed on 27 October 2021).
- Global Invasive Species Database, Species Profile: Polygonum Cuspidatum. Available online: http://www.iucngisd.org/gisd/speciesname/Polygonum+cuspidatum (accessed on 27 October 2021).
- Thompson, J.D.; McNeilly, T.; Gray, A.J. Population variation in Spartina anglica C.E. Hubbard. I. Evidence from a common garden experiment. New Phytol. 1991, 117, 115–128.
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121.
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193.
- Bailey, J.P. The Reproductive Biology and Fertility of Fallopia Japonica (Japanese Knotweed) and its Hybrids in the British Isles. In Ecology and Management of Invasive Riparian Plants; de Waal, C., Child, L.E., Wade, M., Brock, J.H., Eds.; Wiley: Chichester, UK, 1994; pp. 141–158.
- Engler, J.; Abt, K.; Buhk, C. Seed characteristics and germination limitations in the highly invasive Fallopia japonica s.l. (Polygonaceae). Ecol. Res. 2011, 26, 555–562.
- Toews, H.P. Introduction of Native Tree Species in Sites Invaded by Japanese Knotweed Taxa and a Study of Its Affect of the Seedbank; New York State University: New York, NY, USA, 2012; pp. 1–41.
- Bossdorf, O.; Richards, C.L.; Pigliucci, M. Epigenetics for ecologists. Ecol. Lett. 2008, 11, 106–115.
- Schrey, A.W.; Alvarez, M.; Foust, C.M.; Kilvitis, H.J.; Lee, J.D.; Liebl, A.L.; Robertson, M. Ecological epigenetics: Beyond MS-AFLP. Integr. Comp. Biol. 2003, 53, 340–350.
- Douhovnikoff, V.; Dodd, R.S. Epigenetics: A potential mechanism for clonal plant success. Plant Ecol. 2015, 216, 227–233.
- Parepa, M.; Schaffner, U.; Bossdorf, O. Help from under ground: Soil biota facilitate knotweed invasion. Ecosphere 2013, 4, 31.
- Gaskin, J.F.; Schwarzländer, M.; Grevstad, F.S.; Haverhals, M.A.; Bourchier, R.S.; Miller, T.W. Extreme differences in population structure and genetic diversity for three invasive congeners: Knotweeds in western North America. Biol. Invasions 2014, 16, 2127–2136.
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trend. Ecol. Evol. 2002, 17, 164–170.
- Mitchell, C.E.; Power, A.G. Release of invasive plants from fungal and viral pathogens. Nature 2003, 421, 625–627.
- Cappuccino, N.; Carpenter, D. Invasive exotic plants suffer less herbivory than non-invasive plants. Biol. Lett. 2005, 1, 435–438.
- Williams, V.R.J.; Sahli, H.F. A comparison of herbivore damage on three invasive plants and their native congeners: Implications for the enemy release hypothesis. Castanea 2016, 81, 128–137.
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive knotweed affects native plants through allelopathy. Am. J. Bot. 2011, 98, 38–43.
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 2018, 127, 100–109.
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, USA, 1984; pp. 1–422.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578.
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326.
- Qasem, J.R.; Hill, T.A. On difficulties with allelopathy methodology. Weed Res. 1989, 29, 345–347.
- Einhellig, F.A. Interactions involving allelopathy in cropping systems. Agron. J. 1996, 88, 886–893.
- Fuerst, E.P.; Putnam, A.R. Separating the competitive and allelopathic components of interference: Theoretical principles. J. Chem. Ecol. 1983, 9, 937–944.
- Leather, G.R.; Einhelling, F.A. Bioassay of naturally occurring allelochemicals for phytotoxicity. J. Chem. Ecol. 1988, 14, 1821–1828.
- Inderjit; Olosfsdotter, M. Using and Improving Laboratory Bioassays in Rice Allelopathy Research. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 45–55.
- Siemens, T.J.; Blossey, B. An evaluation of mechanisms preventing growth and survival of two native species in invasive Bohemian knotweed (Fallopia × bohemica, Polygonaceae). Am. J. Bot. 2007, 94, 776–783.
- Inderjit; Callaway, R.M. Experimental designs for the study of allelopathy. Plant Soil 2003, 256, 1–11.
- Dommanget, F.; Evette, A.; Spiegelberger, T.; Gallet, C.; Pacé, M.; Imbert, M.; Navas, M.L. Differential allelopathic effects of Japanese knotweed on willow and cottonwood cuttings used in riverbank restoration techniques. J. Environ. Manag. 2014, 132, 71–78.
- Inoue, M.; Nishimura, H.; Li, H.H.; Mizutani, J. Allelochemicals from Polygonum sachalinense Fr. Schm. (Polygonaceae). J. Chem. Ecol. 1992, 18, 1833–1840.
- Vrchotová, N.; Šerá, B. Allelopathic properties of knotweed rhizome extracts. Plant Soil Env. 2008, 54, 301–303.
- Šoln, K.; Žnidaršič, N.; Koce, J.D. Root Growth Inhibition and Ultrastructural Changes in Radish Root Tips After Treatment with Aqueous Extracts of Fallopia Japonica and F. × Bohemica Rhizomes. Protoplasma 2021.
- Šoln, K.; Likar, M.; Koce, J.D. Effects of rhizome extracts from invasive knotweed species Fallopia japonica and F. × bohemica on radish seed germination and root growth of seedlings. Allelopath. J. 2021, 52, 103–118.
- Palmeri, J.; Kiviat, E. Toxic Effects of Knotweed Polygonum cuspidatum s.l. Rhizome on the Mosses Atrichum angustatum and Thuidium Delicatulum. Lindbergia 2021, 1.
- Šerá, B. Effects of soil substrate contaminated by knotweed leaves on seed development. Pol. J. Env. Stud. 2012, 3, 713–717.
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Zákravský, P. Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of dominant native species. NeoBiota 2011, 9, 31–47.
- Heděnec, P.; Novotný, D.; Ust’ak, S.; Honzík, R.; Kovářová, M.; Šimáčková, H.; Frouz, J. Allelopathic effect of new introduced biofuel crops on the soil biota: A comparative study. Eur. J. Soil Biol. 2014, 63, 14–20.
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422.
- Stevens, G.A.; Tang, C.S. Inhibition of seedling growth of crop species by recirculating root exudates of Bidens pilosa L. J. Chem. Ecol. 1985, 11, 1411–1425.
- Mallik, A.U. Allelopathy and competition in coniferous forests. Environ. Sci. 1998, 54, 309–315.
- Kato-Noguchi, H.; Takeshita, S.; Kimura, F.; Ohno, O.; Suenaga, K. A novel allelopathic active substance in Ginkgo biloba. J. Plant Physiol. 2013, 170, 1595–1599.
- Kato-Noguchi, H.; Takeshita, S. Contribution of a phytotoxic compound to the allelopathy of Ginkgo Biloba. Plant Signal. Behav. 2013, 8, e26999.
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelopathic substances of mango (Mangifera indica L.). Weed Biol. Manag. 2020, 20, 131–138.
- Kato-Noguchi, H. Phytotoxic substances involved in teak allelopathy and agroforestry. Appl. Sci. 2021, 11, 3314.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an Invasive plant. Plants 2021, 10, 1028.
- Gerber, E.; Krebs, C.; Murrell, C.; Moretti, M.; Rocklin, R.; Schaffner, U. Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats. Biol. Conserv. 2008, 141, 646–654.
- Urgenson, L.S.; Reichard, S.H.; Halpern, C.B. Community and ecosystem consequences of giant knotweed (Polygonum sachalinense) invasion into riparian forests of western Washington, USA. Biol. Conserv. 2009, 142, 1536–1541.
- Wilson, M.; Freundlich, A.; Martine, C. Understory dominance and the new climax: Impacts of Japanese knotweed (Fallopia japonica) invasion on native plant diversity and recruitment in a riparian woodland. Biodiv. Data J. 2007, 5, e20577.
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443.
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interaction. Science 2003, 301, 1377–1380.
- Pinzone, P.; Potts, D.; Pettibone, G.; Warren, R. Do novel weapons that degrade mycorrhizal mutualisms promote species invasion? Plant Ecol. 2018, 219, 539–548.
- Fan, P.; Hay, A.E.; Marston, A.; Lou, H.; Hostettmann, K. Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. and Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim. Biochem. Syst. Ecol. 2009, 37, 24–34.
- Zubek, S.; Kapusta, P.; Stanek, M.; Woch, M.W.; Błaszkowski, J.; Stefanowicz, A.M. Reynoutria japonica invasion negatively affects arbuscular mycorrhizal fungi communities regardless of the season and soil conditions. Appl. Soil Ecol. 2022, 169, 104152.
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; pp. 1–815.
- Tanner, R.A.; Gange, A.C. The impact of two non-native plant species on native flora performance: Potential implications for habitat restoration. Plant Ecol. 2013, 214, 423–432.
- Zubek, S.; Majewska, M.L.; Błaszkowski, J.; Stefanowicz, A.M.; Nobis, M.; Kapusta, P. Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol. Fertil. Soil 2016, 52, 879–893.
- Hale, A.N.; Kalisz, S. Perspectives on allelopathic disruption of plant mutualisms: A framework for individual- and population-level fitness consequences. Plant Ecol. 2012, 213, 1991–2006.
- Callaway, R.M.; Cipollini, D.; Barto, K.; Thelen, G.C.; Hallett, S.G.; Prati, D.; Stinson, K.; Klironomos, J. Novel weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 2008, 89, 1043–1055.
- Cantor, A.; Hale, A.; Aaron, J.; Traw, M.B.; Kalisz, S. Low allelochemical concentrations detected in garlic mustard-invaded forest soils inhibit fungal growth and AMF spore germination. Biol. Invasions 2021, 13, 3015–3025.
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Engin. 2011, 18, 240–246.
- Lockwood, J.L.; Simberloff, D.; McKinney, M.L.; Von Holle, B. How many, and which, plants will invade natural areas. Biol. Invasions 2001, 3, 1–8.
- Izhaki, I. Emodin: A secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217.
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinefera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86.
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of pterostilbene as a phytoalexin from Vitis vinefera leaves. Phytochemistry 1979, 18, 1025–1027.
- Shi, C.Y.; Ye, Q.B.; Wang, X.H.; Zhang, T.M. Study on the application of Polygonum cuspidatum in “Chinese medince minsterial standards”. Asia Pac. Tradit. Med. 2012, 8, 207–209.
- Bourchier, R.S.; Van Hezewijk, B.H. Distribution and potential spread of Japanese knotweed (Polygonum cuspidatum) in Canada relative to climatic thresholds. Invasive Plant Sci. Manag. 2010, 3, 32–39.
