Periodontitis is a multifactorial chronic inflammatory disease that affects tooth-supporting soft/hard tissues of the dentition. The dental plaque biofilm is considered as a primary etiological factor in susceptible patients; however, other factors contribute to progression, such as diabetes and smoking. Current management utilizes mechanical biofilm removal as the gold standard of treatment. Antibacterial agents might be indicated in certain conditions as an adjunct to this mechanical approach. Studies suggest efficacy in the use of adjunctive antimicrobials in patients with grade C periodontitis of young age or where the associated risk factors are inconsistent with the amount of bone loss present. Meanwhile, alternative approaches such as photodynamic therapy and probiotics showed limited supportive evidence, and more studies are warranted to validate their efficiency.
- antibacterial
- biofilms
- periodontal debridement
- bacterial resistance
- photodynamic therapy
- periodontal disease
- probiotics
- periodontitis
1. Introduction
Periodontitis is an inflammatory disease initiated by dysbiosis of the subgingival microbiome, with aberrant immune response, causing collateral damage to the tooth-supporting tissues and ultimately leading to tooth loss [15,16]. Dental plaque biofilm is considered as the primary etiologic factor for the majority of dental/periodontal diseases [17]. The gold standard of treatment for periodontitis is mechanical debridement of subgingival biofilm. Indeed, suppression of pathogenic microorganisms has for a long time been a keystone in regeneration and repair of periodontal tissues, which can be challenging using mechanical debridement alone, which must be complemented by patient-based plaque control programs [18,19,20]. For many decades, attempts have been made to improve the efficacy of mechanical treatment by introducing different adjuncts such as the use of antimicrobials/antibiotics at different dosages and routes of administration. However, the structural complexity of dental biofilm provides a shelter for many pathogenic microorganisms, making delivery to individual bacteria challenging [21]. In addition, due to the non-specificity of these drugs, they may target useful commensal species which counteract pathogenic biofilm development [22].
2. Structure of Biofilm
3. Management of Dental Biofilm
3.1. Periodontal Debridement: The Gold Standard for Periodontal Therapy
3.2. Adjunctive Systemic and Local Antimicrobials/Antibiotics in Periodontics
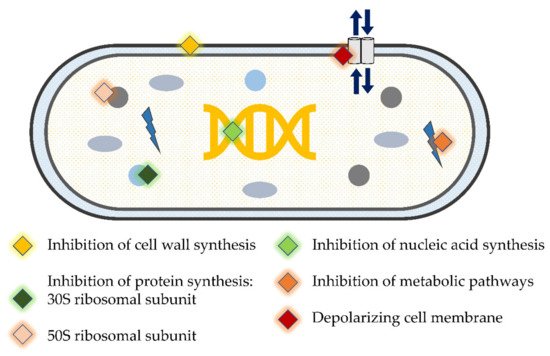
4. Using Antimicrobials/Antibiotics as Adjunct to Periodontal Therapy
A number of studies using various antimicrobials against periodontal pathogens have been carried out. It is apparent that the antimicrobials can kill periodontal pathogens in in vitro biofilm models. However, some studies indicated that amoxicillin (AMX) + metronidazole (MET) were not efficient in reducing the bacterial count [66,67]. Nevertheless, most of the studies consistently reported that the combination of these two antibiotics was superior to using either of them alone, particularly against red complex bacteria [68,69,70,71]. Similar results with regard to these bacteria were obtained with other antibiotics including azithromycin (AZM) [68,71], minocycline [72], and active organic ingredients of mouthrinses [73,74,75]. However, it is important to acknowledge that owing to greater tolerance to antimicrobials, the minimum inhibitory concentration calculated in in vitro studies would purportedly be lower and would bear little relevance to in vivo situations [34,76]. Furthermore, the majority of these studies used laboratory strains in their biofilm models, which apparently differ from clinical strains in their behavior and resistance to antimicrobials [77].
5. Novel Antibacterial Agents and Strategies to Overcome Bacterial Resistance in Dental Biofilm: Pros and Cons
| Author, Year | Study Design, Follow-Up | Study Population | Clinical/Microbiological Parameters | aPDT Treatment Modalities |
|---|---|---|---|---|
| Improvement in microbiological and clinical parameters § | ||||
| Moreira et al., 2015 [118] | Split-mouth RCT, 3-months | Patients with generalized AgP (n = 20) |
|
SD + Diode laser (670 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| Gandhi et al., 2019 [116] | Split-mouth, RCT, 9-months | Periodontitis patients (n = 26) |
|
SD + Diode laser (810 nm)/ICG photosensitizer |
| Annaji et al., 2016 [117] | Split-mouth RCT, 3-months | Patients with AgP (n = 15) |
|
SD+ Diode Laser (810 nm) |
| Wadhwa et al., 2021 [115] | Split-mouth RCT, 6-months | Chronic periodontitis patients (n = 30) | Total viable anaerobic count | SD + Diode laser (810 nm)/ICG photosensitizer |
| Improvement in microbiological parameters only § | ||||
| Muzaheed et al., 2020 [120] | Parallel arm RCT, 3-months | Periodontitis patients (n = 45) |
|
SD + Diode laser (660 nm)/methylene-blue (0.005%) photosensitizer |
| Chondros et al., 2009 [121] | Parallel arm RCT, 6-months | Periodontitis patients (n = 24) |
|
SD + Diode Laser (670 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| No improvement in microbiological and clinical parameters § | ||||
| Chitsazi et al., 2014 [127] | Split-mouth RCT, 3-months | Patients with AgP (n = 24) |
|
SD + Diode Laser (670–690 nm) |
| Rühling et al., 2010 [128] | Parallel arm RCT, 3-months | Periodontitis patients (n = 54) |
|
SD + Diode Laser (635 nm)/5% tolonium chloride photosensitizer |
| Queiroz et al., 2015 [125] Queiroz et al., 2014 [126] |
Parallel arm RCT, 3-months | Periodontitis smoker patients (n = 20) |
|
SD + Diode Laser (660 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| Tabenski et al., 2017 [123] | Parallel arm RCT, 12-months | Periodontitis patients (n = 45) |
|
SD + Diode Laser (670 nm)/phenothiazine chloride photosensitizer |
| Hill et al., 2019 [122] | Split-mouth RCT, 6-months | Periodontitis patients (n = 20) |
|
SD + Diode laser (808 nm)/ICG photosensitizer |
| Pulikkotil et al., 2016 [124] | Split-mouth RCT, 3-months | Periodontitis patients (n = 20) |
|
SD + LED lamp (red spectrum, 628 Hz)/methylene blue photosensitizer |
| Author, Year | Study Design, Follow-Up | Study Population | Strain of Probiotic | Mode/Frequency of Administration | Clinical/Microbiological Parameters |
|---|---|---|---|---|---|
| Improvement in microbiological and clinical parameters § | |||||
| Invernici et al., 2018 [134] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 41) | Bl (HN019) 1 × 109 CFU | Lozenges (10 mg) 2×/day for 30-days |
|
| Invernici et al., 2020 [133] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Bl (HN019) 1 × 109 CFU | Lozenges 2×/day in the morning and before bedtime for 30-days |
|
| Improvement in clinical parameters only § | |||||
| Laleman et al., 2020 [135] | Parallel arm RCT, 6-months | Chronic periodontitis patients (n = 39) | Lr (DSM 17,938 and ATCC PTA 5289) 2 × 108 CFU each | Five probiotic drops applied to residual pocket immediately after SD. Then each patient instructed to use lozenges 2×/day after brushing for 3-months |
|
| Tekce et al., 2015 [137] | Parallel arm RCT, 12-months | Chronic periodontitis patients (n = 30) | Lr (DSM 17,938 and ATCC PTA 5289) 2 × 108 CFU each | Lozenges 2×/day after brushing for 3-weeks |
|
| Improvement in microbiological parameters only § | |||||
| Dhaliwal et al., 2017 [136] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Sf (T-110 JPC), 30 × 107 CFU, Cb (TO-A HIS), 2 × 106 CFU, Bm (TO-A JPC), 1 × 106 CFU and Ls (HIS), 5 × 107 CFU | Bifilac lozenges 2×/day or 21-days |
|
| Teughels et al., 2013 [138] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Lr (DSM17938 and ATCC PTA5289) 9 × 108 CFU each | Lozenges 2×/day for 3-months |
|
| No improvement in microbiological and clinical parameters § | |||||
| Pudgar et al., 2021 [139] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 40) | Lb (CECT7480) and Lp (CECT7481), 6.0 × 109 CFU/mL each | One lozenge/day |
|
| Morales et al., 2018 [140] | Parallel arm RCT, 9-months | Chronic periodontitis patients (n = 47) | Lrh (SP1) 2 × 107 CFU | One sachet in water (150 mL) and ingest it once a day after brushing for 3-months |
|
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11010009
