1. Background
The widespread use of antibiotics for the treatment of various diseases of nonbacterial etiology and other forms of misuse have led to the appearance of bacterial pathogens that are resistant to many well-known antibiotics
[1][2][3][4]. The exceptional evolution of ESKAPE pathogens (
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) has led to the emergence of “superbugs”, which have gained an extraordinary level of resistance
[5][6]. The application of antibiotics has also led to the destruction of the human biota, resulting in reduced immune system activity and facilitating infection
[7]. Annually, around a quarter of all registered deaths across the world are caused by viruses, bacteria and fungi
[8][9]. The above-mentioned problems necessitate a rational approach to the design of a new generation of antibiotics
[10].
Many distinct biocidal agents are known
[11]. Among the most promising and widely studied are cationic surfactants, namely quaternary heteronium salts
[12][13][14]. It is well known that their antimicrobial activity depends on the density of the cationic charge and the hydrophobicity of the active molecule. In the case of cationic surfactants, the determinative factors are the charge, the number of cationic centers, the nature of the counter-ion, and the length/number of alkyl substituents and aromatic groups
[15]. These structure features regulate the self-organization of the surfactants, increasing the local concentrations and cationic charges of amphiphiles on bacteria. The so-called “cut-off effect” in a homologous series of surfactants with long hydrocarbon chains should be noted: as the chain length increases, the biological activity of the compounds increases up to a critical point, beyond which the activity is lost
[16].
An equally important aspect of the biological properties of new compounds is cytotoxicity
[17]. The balance between strong antimicrobial properties and low toxicity to normal cells is the basis of the search for new effective antimicrobial agents. Compounds with strong antimicrobial effects cannot be approved if they exhibit high cytotoxicity to healthy eukaryotic cells.
Among the most prevalent bactericides are various quaternary ammonium salts (QASs)
[18]. They are also active against bacteria, fungi, parasites and even lipophilic viruses
[19]. Generally, they disrupt the cell membranes of micro-organisms
[20][21]. The long lipophilic substituent and the charged center of QAS allow it to become incorporated into the phospholipid bilayer, destroying the cell membrane and acting on bacteria regardless of the species. Therefore, QASs are active against multidrug-resistant strains such as
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii and
Pseudomonas aeruginosa [6]. Some additional possible mechanisms of the influence of biocides on the pathogenic cells are proposed
[22].
Quaternary phosphonium salts (QPSs) are similar in their structure and biological activity against microbes
[23][24]. Their bactericidal activity and safety for humans were discovered in the middle of the 20th century
[25] and continue to be studied
[26]. Particularly intensive research on their biological activity has been spurred by the spread of the usage of phosphonium ionic liquids (PILs) in science and technology. Seddon et al. studied the antimicrobial activity of the series of tri-
n-hexyl(R)phosphonium salts with even numbers of carbon atoms (from C2 to C16) in the fourth alkyl substituent of normal structure
[27]. It was found that the alkyl substituent length was responsible for the antimicrobial properties of PILs. The effect of the nature of the anion on the antimicrobial activity of the phosphonium compound was investigated in the example of tri-
n-hexyl(
n-tetradecyl)phosphonium salts. The results were compared to those of benzalkonium chloride for a broad spectrum of micro-organisms: cocci, bacilli, rods and fungi.
Another study showed that tri-
n-hexyl(
n-tetradecyl)phosphonium salts that were coupled with various anions met all the requirements of new compounds for pharmaceutical use. Their antibacterial, anticancer and antioxidant activities were also demonstrated
[28].
Phosphonium salts based on triphenylphosphine have been studied to a greater extent. Apart from a broad spectrum of biological activity, they exhibit strong antifouling properties
[29]. Various triphenyl(R)phosphonium compounds have demonstrated biological activity: naphtoquinone derivatives
[30], carboxylated betaines with an alkyl substituent
[31] and metronidazole derivatives
[32]. One of the areas of research into the biological activity of QPSs is the study of dicationic compounds based on bis(triphenylphosphonium) salts
[17][33]. Zhang et al. showed the higher antibacterial activity of dicationic compared to monocationic salts.
It should be mentioned that phosphonium compounds have also been applied as bactericides that are covalently bonded to plastic surfaces or used as co-extrusive additives for plastic materials in goods production
[34]. Such surfaces are confirmed to demonstrate antibacterial properties. Another work studied a plastic surface with a bonded phosphonium salt containing an oxanorbornene moiety
[35]. Of the various compounds, tri-
p-methoxyphenylphosphine was the most active against bacteria, but it also demonstrated the highest hemotoxicity.
Quaternary phosphonium salts act as potential antimicrobial agents with a number of notable benefits
[36]. However, the systematic study of the structure–property relationships and the development of a strategy for the regulation of biological properties through the structural tuning of active molecules is still required. Notably, the key to the optimization of potential antimicrobial agents remains the balance between antimicrobic activity and cytotoxicity. The peculiar structure of the sterically hindered salt cation, its charge distribution and its shielding of charged phosphorus atoms by
tert-butyl groups differ greatly from the classical quaternary heteronium salts, allow the hypothesis of distinctive biological properties and different mechanisms of interaction with living cells
[37]. Nowadays, a few sterically hindered QPSs are known
[38][39] and their biological activity is not studied.
2. Antimicrobial and Fungicidal Activity
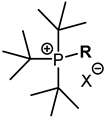
Tri-tert-butyl(n-alkyl)phosphonium salts contain a sterically hindered head group with a cationic charge on the phosphorus atom and a linear aliphatic “tail” that imparts hydrophobic properties to the compounds. Halide ions and a weakly coordinating tetrafluoroborate anion were used as counter-ions. The structures and designations of the QPSs are summarized in Table 1. The first members of the QPSs 1a,b–3a,b are high-melting-point white powders; most have melting points around 100–120 °C. It should be noted that the salts 11a–17a are ‘ionic liquids’ in terms of the classical definition and melt below 100 °C. With an increase in the length of the alkyl substituent, the hydrophobicity of the molecules increases. QPSs with halide anions are highly soluble in water, although their solubility decreases with an increase in molecular weight. Conversely, the phosphonium tetrafluoroborates are almost insoluble in water, with the first five members being weakly soluble exceptions (1b–5b). To increase their solubility, a 5% aqueous solution of DMSO was used.
Table 1. The structure of the QPSs under investigation.
 |
| R |
X = I, Br |
X = BF4 |
| CH3 |
1a * |
1b |
| C2H5 |
2a * |
2b |
| n-C3H7 |
3a * |
3b |
| n-C4H9 |
4a |
4b |
| n-C5H11 |
5a |
5b |
| n-C6H13 |
6a |
6b |
| n-C7H15 |
7a |
7b |
| n-C8H17 |
8a |
8b |
| n-C9H19 |
9a |
9b |
| n-C10H21 |
10a |
10b |
| n-C11H23 |
11a |
11b |
| n-C12H25 |
12a |
12b |
| n-C13H27 |
13a |
13b |
| n-C14H29 |
14a |
14b |
| n-C15H31 |
15a |
15b |
| n-C16H33 |
16a |
16b |
| n-C17H35 |
17a |
17b |
| n-C18H37 |
18a |
18b |
| n-C20H41 |
20a |
20b |
The synthesized compounds were tested for antimicrobial activity against a number of Gram-positive bacteria, Staphylococcus aureus (Sa), Bacillus cereus (Bc) and Enterococcus faecalis (Ef), and Gram-negative bacteria, Escherichia coli (Ec) and Pseudomonas aeruginosa (Pa), including the methicillin-resistant strains S. aureus MRSA-1 (resistant to fluoroquinolones and beta-lactams) and MRSA-2 (resistant to beta-lactams). The methicillin-resistant strains S. aureus were transferred by the Republican Clinical Hospital of the Republic of Tatarstan (Kazan). The fungicidal activity against Candida albicans (Ca) and Trichophyton mentagrophytes var. gypseum (Tm) was studied. These pathogens were chosen because they are the most widespread in the food industry and the healthcare system.
The results are shown in
Table 2 (and
Table S1 in the Supplementary Materials for QPSs with 1–9 carbon atoms in the alkyl substituent). The compounds
1a,b–5a,b do not possess antimicrobial activity; the minimum inhibitory concentrations (MICs) of these compounds exceed 500 μg/mL, which is quite likely due to the short length of their alkyl substituents and their consequent low lipophilicity, rendering them ineffective. With an increase in the lipophilicity of the alkyl substituent, the ionic compounds
6a,b–18a,b and
20a,b start to show antimicrobial activity that reaches a maximum for
11a,b–18a,b. The MICs of the last compounds against Gram-positive bacteria (
S. aureus, B. cereus and
E. faecalis) were in the range of 0.12–0.98 μg/mL, which is at the level of the widely used antibiotic of the fluoroquinolone series, ciprofloxacin. It should be noted that these QPSs also demonstrated high antimicrobial activity against
MRSA strains.
Table 2. (a) Antimicrobial and fungicidal activity of QPSs: minimum inhibitory concentrations. (b) Antimicrobial and fungicidal activity of QPSs: minimum bactericidal and fungicidal concentrations.
| (a) |
| Compound |
Minimum Inhibitory Concentration (MIC), µg/mL |
| Sa |
Bc |
Ef |
MRSA-1 |
MRSA-2 |
Ec |
Pa |
Tm |
Ca |
| 10a |
1.9 ± 0.1 |
3.9 ± 0.2 |
7.8 ± 0.6 |
1.9 ± 0.1 |
3.9 ± 0.2 |
31.3 ± 2.7 |
- |
125 ± 11 |
31.3 ± 2.6 |
| 10b |
3.9 ± 0.2 |
7.8 ± 0.6 |
15.6 ± 1.3 |
1.9 ± 0.1 |
3.9 ± 0.2 |
62.5 ± 5.4 |
- |
125 ± 10 |
31.3 ± 2.4 |
| 11a |
0.9 ± 0.07 |
1.9 ± 0.1 |
7.8 ± 0.5 |
1.9 ± 0.1 |
0.9 ± 0.08 |
31.3 ± 2.6 |
- |
31.3 ± 2.5 |
62.5 ± 5.5 |
| 11b |
0.9 ± 0.07 |
1.9 ± 0.1 |
7.8 ± 0.6 |
0.3 ± 0.02 |
0.5 ± 0.04 |
62.5 ± 5.3 |
- |
125 ± 9 |
31.3 ± 2.4 |
| 12a |
0.5 ± 0.03 |
1.9 ± 0.01 |
0.9 ± 0.06 |
0.5 ± 0.03 |
0.5 ± 0.03 |
7.8 ± 0.6 |
62.5 ± 5.5 |
15.6 ± 1.2 |
15.6 ± 1.4 |
| 12b |
0.5 ± 0.03 |
1.9 ± 0.01 |
1.9 ± 0.02 |
1.9 ± 0.1 |
0.5 ± 0.04 |
15.6 ± 1.3 |
125 ± 11 |
31.3 ± 2.4 |
15.6 ± 1.3 |
| 13a |
0.2 ± 0.01 |
0.9 ± 0.07 |
3.9 ± 0.3 |
0.5 ± 0.04 |
0.3 ± 0.02 |
3.9 ± 0.02 |
15.6 ± 1.3 |
125 ± 10 |
15.6 ± 1.2 |
| 13b |
0.2 ± 0.01 |
0.5 ± 0.04 |
1.9 ± 0.1 |
0.3 ± 0.02 |
0.3 ± 0.01 |
7.8 ± 0.6 |
31.3 ± 2.3 |
62.5 ± 5.2 |
7.8 ± 0.5 |
| 14a |
1.9 ± 0.2 |
7.8 ± 0.6 |
1.9 ± 0.1 |
0.5 ± 0.03 |
0.5 ± 0.04 |
1.9 ± 0.1 |
15.6 ± 1.1 |
125 ± 11 |
7.8 ± 0.6 |
| 14b |
0.1 ± 0.008 |
0.9 ± 0.07 |
0.9 ± 0.08 |
0.3 ± 0.01 |
0.3 ± 0.02 |
1.9 ± 0.1 |
7.8 ± 0.6 |
15.6 ± 1.3 |
3.9 ± 0.3 |
| 15a |
0.2 ± 0.01 |
0.5 ± 0.03 |
0.5 ± 0.03 |
0.5 ± 0.02 |
0.5 ± 0.04 |
1.9 ± 0.1 |
7.8 ± 0.7 |
62.5 ± 5.3 |
1.9 ± 0.1 |
| 15b |
0.2 ± 0.01 |
0.5 ± 0.03 |
0.5 ± 0.04 |
0.3 ± 0.01 |
0.5 ± 0.03 |
3.9 ± 0.2 |
3.9 ± 0.2 |
62.5 ± 5.5 |
1.9 ± 0.1 |
| 16a |
0.5 ± 0.04 |
1.9 ± 0.1 |
0.5 ± 0.03 |
1.9 ± 0.1 |
0.5 ± 0.03 |
0.9 ± 0.07 |
31.3 ± 2.7 |
- |
1.9 ± 0.1 |
| 16b |
0.5 ± 0.03 |
1.9 ± 0.1 |
0.5 ± 0.04 |
1.9 ± 0.1 |
0.5 ± 0.03 |
1.9 ± 0.1 |
3.9 ± 0.02 |
62.5 ± 5.6 |
3.9 ± 0.2 |
| 17a |
0.2 ± 0.01 |
0.9 ± 0.07 |
1.9 ± 0.1 |
0.9 ± 0.06 |
0.9 ± 0.07 |
15.6 ± 1.2 |
7.8 ± 0.5 |
125 ± 10 |
1.9 ± 0.1 |
| 17b |
0.5 ± 0.04 |
1.9 ± 0.1 |
0.5 ± 0.03 |
1.9 ± 0.1 |
1.9 ± 0.1 |
15.6 ± 1.2 |
7.8 ± 0.6 |
125 ± 10 |
1.9 ± 0.1 |
| 18a |
0.9 ± 0.08 |
7.8 ± 0.6 |
0.9 ± 0.07 |
0.9 ± 0.07 |
0.9 ± 0.07 |
15.6 ± 1.3 |
15.6 ± 1.2 |
125 ± 11 |
1.9 ± 0.1 |
| 18b |
1.9 ± 0.1 |
31.3 ± 2.2 |
1.9 ± 0.1 |
7.8 ± 0.6 |
3.9 ± 0.2 |
15.6 ± 1.3 |
15.6 ± 1.3 |
250 ± 19 |
7.8 ± 0.7 |
| 20a |
3.9 ± 0.2 |
15.6 ± 1.3 |
3.9 ± 0.2 |
7.8 ± 0.6 |
1.9 ± 0.1 |
62.5 ± 5.5 |
31.3 ± 2.4 |
31.3 ± 2.3 |
31.3 ± 2.5 |
| 20b |
3.9 ± 0.2 |
62.5 ± 5.5 |
7.8 ± 0.6 |
15.6 ± 1.3 |
7.8 ± 0.6 |
62.5 ± 5.7 |
125 ± 11 |
250 ± 18 |
15.6 ± 1.3 |
| Ciprofloxacin |
0.5 ± 0.03 |
0.5 ± 0.04 |
3.9 ± 0.3 |
125 ± 11 |
0.9 ± 0.07 |
0.5 ± 0.03 |
0.5 ± 0.03 |
|
|
| Ketoconazole |
|
|
|
|
|
|
|
3.9 ± 0.2 |
3.9 ± 0.3 |
| (b) |
| Compound |
Minimum Bactericidal and Fungicidal Concentration (MBC, MFC), µg/mL |
| Sa |
Bc |
Ef |
MRSA-1 |
MRSA-2 |
Ec |
Pa |
Tm |
Ca |
| 10a |
1.9 ± 0.1 |
31.3 ± 2.5 |
7.8 ± 0.6 |
15.6 ± 1.2 |
3.9 ± 0.2 |
31.3 ± 2.6 |
- |
125 ± 11 |
31.3 ± 2.3 |
| 10b |
3.9 ± 0.2 |
125 ± 9 |
15.6 ± 1.2 |
15.6 ± 1.3 |
7.8 ± 0.6 |
62.5 ± 5.5 |
- |
125 ± 10 |
31.3 ± 2.2 |
| 11a |
1.9 ± 0.1 |
125 ± 10 |
7.8 ± 0.6 |
1.9 ± 0.1 |
7.8 ± 0.6 |
31.3 ± 2.3 |
- |
31.3 ± 2.5 |
62.5 ± 5.7 |
| 11b |
3.9 ± 0.2 |
125 ± 10 |
15.6 ± 1.2 |
0.5 ± 0.03 |
0.9 ± 0.07 |
62.5 ± 5.4 |
- |
125 ± 9 |
31.3 ± 2.1 |
| 12a |
0.5 ± 0.03 |
62.5 ± 5.3 |
1.9 ± 0.1 |
0.5 ± 0.04 |
7.8 ± 0.6 |
7.8 ± 0.7 |
125 ± 11 |
15.6 ± 1.3 |
31.3 ± 2.7 |
| 12b |
0.5 ± 0.03 |
62.5 ± 5.5 |
15.6 ± 1.3 |
0.5 ± 0.04 |
31.3 ± 2.7 |
15.6 ± 1.2 |
125 ± 10 |
62.5 ± 5.2 |
15.6 ± 1.3 |
| 13a |
0.5 ± 0.04 |
7.8 ± 0.6 |
7.8 ± 0.6 |
0.3 ± 0.02 |
0.5 ± 0.03 |
3.9 ± 0.2 |
31.3 ± 2.4 |
125 ± 10 |
31.3 ± 2.2 |
| 13b |
0.2 ± 0.01 |
31.3 ± 2.3 |
3.9 ± 0.3 |
0.5 ± 0.03 |
3.9 ± 0.3 |
7.8 ± 0.6 |
31.3 ± 2.2 |
62.5 ± 5.3 |
7.8 ± 0.6 |
| 14a |
1.9 ± 0.1 |
15.6 ± 1.3 |
3.9 ± 0.2 |
7.8 ± 0.6 |
62.5 ± 5.3 |
1.9 ± 0.1 |
125 ± 11 |
125 ± 10 |
15.6 ± 1.2 |
| 14b |
1.9 ± 0.1 |
15.6 ± 1.2 |
1.9 ± 0.1 |
0.5 ± 0.03 |
0.5 ± 0.03 |
3.9 ± 0.2 |
62.5 ± 5.4 |
15.6 ± 1.3 |
15.6 ± 1.2 |
| 15a |
0.5 ± 0.03 |
15.6 ± 1.3 |
1.9 ± 0.1 |
0.5 ± 0.04 |
0.5 ± 0.03 |
3.9 ± 0.2 |
7.8 ± 0.6 |
62.5 ± 5.7 |
3.9 ± 0.2 |
| 15b |
0.5 ± 0.03 |
15.6 ± 1.3 |
3.9 ± 0.2 |
0.3 ± 0.01 |
0.5 ± 0.04 |
3.9 ± 0.2 |
3.9 ± 0.3 |
62.5 ± 5.2 |
15.6 ± 1.2 |
| 16a |
0.5 ± 0.03 |
31.3 ± 2.2 |
0.5 ± 0.03 |
7.8 ± 0.6 |
7.8 ± 0.7 |
1.9 ± 0.1 |
125 ± 9 |
- |
3.9 ± 0.3 |
| 16b |
0.5 ± 0.03 |
31.3 ± 2.4 |
0.5 ± 0.03 |
15.6 ± 1.2 |
3.9 ± 0.2 |
1.9 ± 0.1 |
62.5 ± 5.3 |
62.5 ± 5.5 |
3.9 ± 0.2 |
| 17a |
0.5 ± 0.03 |
15.6 ± 1.2 |
1.9 ± 0.1 |
0.9 ± 0.07 |
0.9 ± 0.06 |
15.6 ± 1.3 |
7.8 ± 0.7 |
125 ± 10 |
3.9 ± 0.2 |
| 17b |
0.5 ± 0.04 |
15.6 ± 1.3 |
3.9 ± 0.2 |
1.9 ± 0.1 |
1.9 ± 0.1 |
7.8 ± 0.6 |
15.6 ± 1.3 |
125 ± 11 |
7.8 ± 0.7 |
| 18a |
0.9 ± 0.07 |
31.3 ± 2.5 |
0.9 ± 0.07 |
15.6 ± 1.2 |
0.9 ± 0.07 |
15.6 ± 1.1 |
62.5 ± 5.6 |
125 ± 9 |
3.9 ± 0.2 |
| 18b |
3.9 ± 0.2 |
125 ± 11 |
3.9 ± 0.2 |
15.6 ± 1.2 |
3.9 ± 0.3 |
62.5 ± 5.4 |
31.3 ± 2.5 |
250 ± 19 |
15.6 ± 1.2 |
| 20a |
3.9 ± 0.2 |
31.3 ± 2.6 |
7.8 ± 0.6 |
31.3 ± 2.7 |
15.6 ± 1.2 |
62.5 ± 5.5 |
125 ± 11 |
31.3 ± 2.5 |
62.5 ± 5.7 |
| 20b |
3.9 ± 0.3 |
- |
15.6 ± 1.3 |
15.6 ± 1.2 |
7.8 ± 0.7 |
62.5 ± 5.5 |
- |
250 ± 18 |
31.3 ± 2.1 |
| Ciprofloxacin |
0.5 ± 0.03 |
0.5 ± 0.04 |
3.9 ± 0.3 |
250 ± 19 |
0.9 ± 0.06 |
0.5 ± 0.03 |
0.5 ± 0.03 |
|
|
| Ketoconazole |
|
|
|
|
|
|
|
3.9 ± 0.2 |
3.9 ± 0.3 |
Compounds 14a,b–16a,b were the most active against the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa. The antifungal activity was most pronounced for compounds 15a,b–18a,b and manifested at the level of the reference drug ketoconazole. Interestingly, most of the leading compounds acted both bactericidally and fungicidally; their MICs and MBCs (MFCs) differed from each other by no more than four times. The difference in antimicrobial activity between halide and tetrafluoroborate anions was minor, affirming the determining role of the cationic nature in the biological activity of this comparison series.
It should be mentioned that the “cut-off effect”—whereby, with an increase in the number of methylene fragments in the alkyl substituent, the compounds increase in biological activity up to a critical point, beyond which they cease to be effective—was not observed in the range of sterically hindered phosphonium salts that was studied. The biocidal activity of QPSs with alkyl chain lengths of 18 and 20 carbon atoms was lower, but these compounds were still active against bacteria and fungi.
3. Hemolytic and Cytotoxic Activity of QPSs
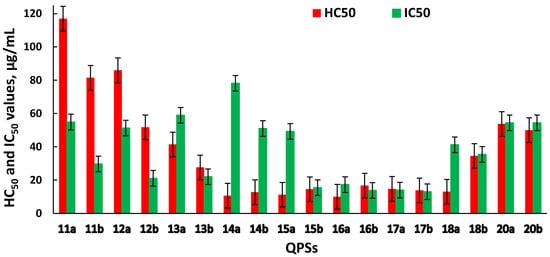
An important characteristic when assessing the biological activity of new chemical compounds is their cytotoxic effect on eukaryotic cells. The ability of an investigated compound to cause the destruction of human erythrocytes illustrates its toxic effect on the internal environment. The hemolysis assay is a simple screening test that can help in the study of cytotoxicity in more complex models. Cell lines obtained from various human organs and tissues that allow for adequate estimation of the effects of new potential drugs on cell metabolism can serve as experimental models. Hence, the sterically hindered QPSs were tested for cytotoxicity against red blood cells and the human hepatocyte Chang liver cell line (Figure 1).
Figure 1. Hemotoxic and cytotoxic activity of QPSs, expressed in terms of HC50 and IC50.
Data on hemolytic and cytotoxic activity are represented by the HC50 (the concentration of the test compound that causes the 50% hemolysis of erythrocytes in the experiment) and IC50 (the concentration of the test compound that causes the death of 50% of the cells in the test population) values, respectively.
Compounds
1a,b–10a,b, across the tested range of concentrations, did not exhibit high hemolytic or cytotoxic activity, with HC
50 > 125 µg/mL and IC
50 ranging from 66 to >100 µg/mL (
Table S2 in Supplementary Materials).
It was found that the manifestation of hemolytic activity by the tested compounds depended on the cation structure, while the anion nature did not dramatically influence the hemotoxicity (Figure 1). The compounds 11a,b–13a,b exhibited weaker hemotoxic properties, while their antibacterial activity was still very high. With an increase in the lipophilicity of the alkyl substituents (14a,b–17a,b), the hemolytic effect slightly decreased. However, at the same time, there was an increase in antibacterial and antifungal activity in a number of cases. A further increase in the alkyl substituent length (18b, 20a,b) led to a decrease in both hemotoxicity and antimicrobial activity. The exception was compound 18a, for which the hemolytic properties of which did not change, and high antibacterial and antifungal activity was demonstrated.
Against normal liver cells (Chang liver), the compounds 11a–15a were observed to be less toxic than their counterparts with tetrafluoroborate anion. Apparently, in this case, the change in cytotoxic properties was associated with the nature of the counter-ion. Meanwhile, a dependence on the anion nature for phosphonium compounds 16a,b–18a,b and 20a,b was not pronounced. In some cases, the HC50 and IC50 values for compounds with different counter-ions were similar; however, in other examples, they differed by about 5–7 times. Compounds 12a and 13a should be especially noted, as they showed the lowest cytotoxicity against erythrocytes and human liver cells and, at the same time, very high antimicrobial activity against the tested strains of micro-organisms, including MRSA.
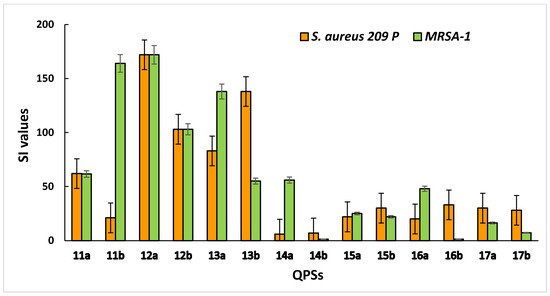
The selectivity of compounds for microbial cells is an important criterion for assessing the cytotoxic effects. This indicator is characterized by the selectivity index (SI), which was calculated as the ratio between the HC50 value for erythrocytes (IC50 for eukaryotic cells) and the MBC value for bacterial cells. It was found that compounds 12a,b and 13a,b showed the highest selectivity for the S. aureus 209 P and MRSA-1 strains, with low toxicity to human blood and liver cells (Figure 2).
Figure 2. Selectivity of QPSs for bacteria (S. aureus 209 P and MRSA-1) compared to red blood cells.
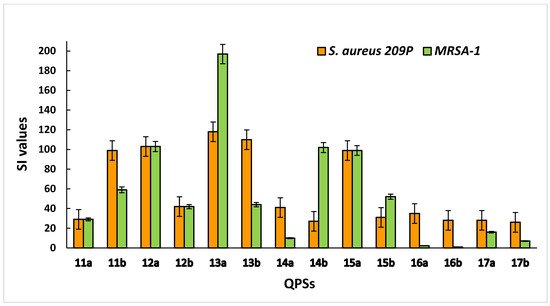
For liver cells, the highest selectivity was shown by compounds 12a, 13a and 15a (Figure 3). Overall, most of the studied QPSs showed outstanding selectivity for the S. aureus 209 P and MRSA strains while demonstrating low toxicity to human erythrocytes and hepatocytes.
Figure 3. Selectivity of QPSs for bacteria (S. aureus 209 P and MRSA-1) compared to Chang liver cells.
The results obtained for the investigated phosphonium salts demonstrates their high antimicrobial activity and selectivity for microbes as well as their safety for normal human cells, which is a prerequisite for the development of potential new antimicrobial agents.
4. Analysis of CV Absorbance Spectra
The study of the mechanism of action is one of the most important stages in the investigation of new antimicrobial agents. According to a number of authors, the mechanisms of action of QPSs could be associated with the disruption of the structure of the bacterial cytoplasmic membrane, leading to a change in its permeability
[40].
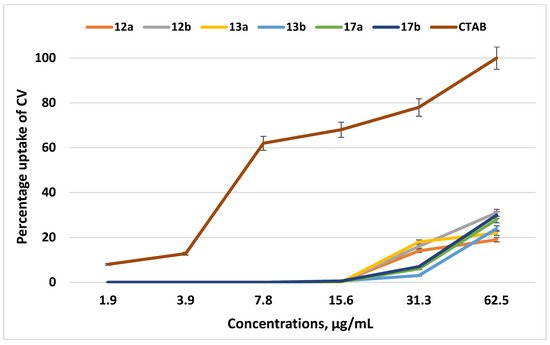
Therefore, we studied the membranotropic effect of the QPSs on the absorption of the crystal violet (CV) dye, since an increase in the ability of the CV dye to penetrate implies a change in the permeability of the cytoplasmic membrane (BMC). The experiment was carried out for the examples of the lead compounds 12a,b and 13a,b; QPSs with longer alkyl substituents, 17a,b, were also chosen for comparison. The traditional membranotropic agent cetyl(trimethyl)ammonium bromide (CTAB) was used as a control. Figure 4 demonstrates that all of the tested compounds in the ranges of their MICs and MBCs (1.9–62.5 μg/mL) had very poor effects on the permeability of the cytoplasmic membrane of S. aureus 209 P. At the highest concentrations of the lead compounds, which were many times higher than the MICs and MBCs, the uptake of CV did not exceed 30%.
Figure 4. The percentage of crystal violet in S. aureus 209 P supernatant after 30 min incubation with various concentrations (µg/mL) of the test compounds and CTAB. The optical density of the sample at a wavelength of 540 nm with the dye in the absence of cells was taken as 100%.
It should be noted that the tested compounds, throughout the ranges of studied concentrations, did not have a significant effect on the structure of the cytoplasmic membrane of
S. aureus 209 P compared to the classic CTAB surfactant. In this regard, one can assume that the specific mechanisms of action of the tested compounds in the concentration ranges, corresponding to the MICs and MBCs, differ from the actions of classical cationic surfactants and could be associated with their ability to disrupt the energy metabolism in bacteria due to the induction of a sharp decrease in the membrane potential by stimulating protonophoric uncoupling
[41]. The difference of tri-
tert-butyl(
n-alkyl)phosphonium salts from classical biocides based on non-hindered heteronium salts could consist of the structure, arrangement and charge distribution of the cation.
5. Antimicrobial Coating of 3D-Printed Polymeric Surfaces
There are incredible advantages to using 3D-printing technology for creating customized products with precisely defined parameters. In many cases, biocidal properties are necessary for such products, but they are not limited to uses in medicine
[42][43][44][45]. Imparting antimicrobial properties to surfaces, as well as preventing the formation of biofilms and their fouling by resident living multicellular organisms, is of great practical importance. There are several approaches to imparting bactericidal properties to surfaces: the physical modification of the surface, the use of additives in the material
[46], or the attachment of biocides to the surface
[47]. The most affordable, available and effective method is the surface treatment of finished products. This method, in contrast to the use of co-extrusive additives, does not change the construction properties of the materials and does not require incorporation into the production process.
There are two types of coatings. For the first, the coating is covalently bonded to the protected surface, and in the second case, the biocide-release mechanism is implemented when biocide not only acts on the surface but also creates an inhibition zone
[47].
In order to study the possibility of using QPSs as surface biocides for FDM polymers, the six materials most commonly used for 3D printing (Table 3) were coated with the lead compound 12a and subjected to testing.
Table 3. Polymers for 3D printing.
| FDM Polymers |
Abbreviation |
| acrylonitrile butadiene styrene |
ABS |
| polylactic acid |
PLA natural |
| polylactic acid modified |
PLA+ |
| polyethylene terephthalate glycol |
PETG |
| polycarbonate modified |
PC+ |
| nylon |
Nylon |
The plastic samples, which were 10 mm × 10 mm × 2 mm, were preliminary sterilized and placed into an ethanolic solution of
12a for 1 min. Then, the studied and control (without coating) samples were immersed in bacterial suspensions with a known concentration of bacteria (10
5–10
8 bacteria/mL) for 24 h, and the number of viable micro-organisms was then estimated
[48]. The results are shown in
Table 4.
Table 4. Evaluation of bactericidal properties of a plastic sample (1 cm2) after 24 h of incubation with 105 S. aureus 209 P.
| Concentration of 12a, % |
CFU/cm2 |
| Name of Plastic Samples |
| ABS |
PLA Natural |
PLA+ |
PETG |
PC+ |
Nylon |
| 0 a |
1.3 × 104 |
4.0 × 104 |
8.0 × 104 |
5.0 × 104 |
3.6 × 104 |
4.5 × 104 |
| 0.01 |
- |
- |
- |
- |
- |
2.0 × 102 |
| 0.001 |
2.5 × 103 |
5.0 × 103 |
1.6 × 104 |
1.8 × 103 |
1.2 × 104 |
3.2 × 104 |
The highest bactericidal effect for 12a was achieved on the surfaces of ABS, PLA natural and PETG plastics (Table 4). At a concentration of 0.01%, a complete absence of bacterial growth was observed. Upon reducing the concentration of the compound to 0.001%, the best bactericidal effect was observed for PETG transp. The bacterial cell concentration was decreased by 27 times compared to the control.
The present experiment is the first step in developing potential QPSs for the coating of plastic products. Further investigation would include the addition of phosphonium compounds to the plastic mass to provide it with biocidal properties in order to obtain antiseptic materials.
6. Zone-of-Inhibition Test
The inhibition-zone test is a quick and qualitative way to measure the ability of an antimicrobial agent to suppress microbial growth
[49]. The sensitivity of the method is quite high and even a small amount of residual biocide in a model system can be detected. Thus, to prove that the antimicrobial properties of the plastic samples under study were only manifested by contact with the surface, excluding the slow release of biocidal compounds, the PC+ plastic sample was examined in a zone-of-inhibition test. In this experiment, we used cultures of the bacteria
P. aeruginosa and
S. aureus. First, the microbial suspension was spread on a sterile agar plate. Then, a plastic sample (PC+), which was preliminarily immersed for 1 min in a 1% solution of compound
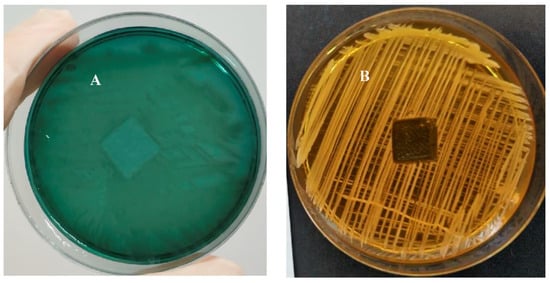
12a in ethanol, was placed in the center of the Petri dish with agar. During the incubation period, the inoculum bacteria can only grow where is no antimicrobial agent. If the active agent is washed out of the sample into the environment (in this case, agar), bacterial growth in the area around the sample is suppressed. The width of this area is determined by the diffusion of the active compound in the vicinity. As can be seen in
Figure 5, testing the sample on both bacterial cultures displayed no zones of inhibition, indicating no evidence of the biocidal release of
12a from the PC+ surface. This suggests that the antimicrobial action of the tested plastic materials is only manifested by contact with the surface. From the results obtained, it is clear that a relatively stable surface coating was achieved using a simple experimental procedure.
Figure 5. Picture of a zone-of-inhibition-test result of 1% 12a coating plastic sample (PC+); (A) zone of inhibition of P. aeruginosa and (B) zone of inhibition of S. aureus.