Paraoxone as an organophosphorus compound has nephrotoxic properties for mammalian kidneys. In experiments on rats, even a single intoxication with paraoxone leads to the development of changes in the biochemical parameters of blood and urine, and causes structural changes in the renal tubules and ultrastructural changes in the glomerular basal membrane.
- nephrotoxicity
- rats
- paraoxon
- biomarkers
- calbindin
- chondroitin sulphate
- creatinine clearance
1.Nephrotoxicity of organophosphate compounds
1.1.Paraoxone nephrotoxicity
2. Animal Models of Acute paraoxone Intoxication
The main biochemical characteristic of rodents is the presence of carboxylesterase in plasma, which is a target for OPs and can significantly distort their specific effects. To eliminate the effects of carboxylesterase in the first model (M1, POX2x group), POX was administered twice in doses of 110 g/kg and 130 g/kg subcutaneously, at 1 h intervals. In the second model (M2, CBPOX group), 1 h before POX poisoning at a dose of 130 g/kg subcutaneously, carboxylesterase activity was pre-inhibited by administration of the specific inhibitor cresylbenzodioxaphosphorin oxide (CBDP, 3.3 mg/kg intraperitoneally). In the third model (M3), POX was administered subcutaneously once at doses of LD16 (241 g/kg), LD50 (250 g/kg) and LD84 (259 g/kg).
2.1 Acute nephrotoxicity of paraoxone (POX) for rats
2.1.1. Plasma Biochemistry
Microscopic examination of rat urine sediment in all models of acute paraoxon poisoning showed no evidence of acute kidney damage, including haematuria, erythrocyturia, pyuria, crystalluria and cylinduria as well as increased epithelial cell count. The only significant difference in urine biochemistry of poisoned rats was a marked glucosuria observed 24 h after the poisoning in both POX2x and CBPOX groups.
2.1.3. Chondroitin Sulphate (CS) in Rat Urine
2.1.4. Calbindin, KIM-1, TIMP-1 in Rat Urine
| Control | POX2x | CBPOX | |
|---|---|---|---|
| Calbindin | 4101 ± 334 | 6419 ± 1201 | 7383 ± 990 * |
| KIM-1 | 21.8 ± 5.5 | 28.8 ± 6.0 | 24.1 ± 6.9 |
| TIMP-1 | 32,755 ± 7830 | 13,078 ± 9771 | 19,174 ± 11,596 |
* p ≤ 0.05; Results are presented in ng/mol_creatinine.
Currently, calbindin is considered as an informative biomarker of damage of distal tubules and collecting tubes of the kidneys [59,60]. Changes in the morphometric characteristics of the tubule epithelium in 1–3 days after poisoning, and increased calbindin excretion, indicates tubule damage.
2.1.5. Histopathological Changes in the Kidneys
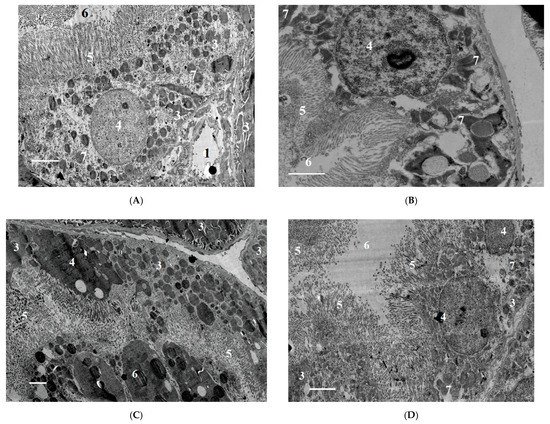
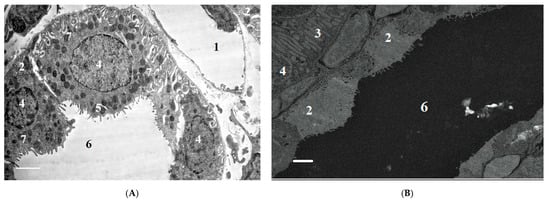
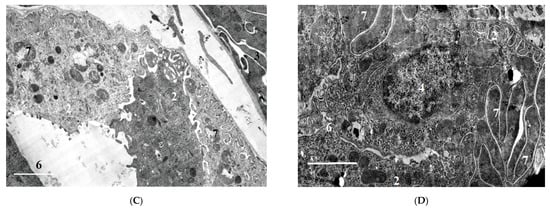
2.1.6. Ultrastructural Changes in Rat Kidneys after the Poisoning
3. Conclusions
The nephrotoxic effects of POX in rats occur regardless of the mode of prior inhibition of carboxylesterase activity. The use of a conventional model of poisoning at a single dose of LD16 does not lead to the development of any abnormalities characterizing the nephrotoxicity of POX at this dose. At the same time, POX at the doses of LD50 and LD84 causes marked ultrastructural changes in the cells of tubules, which is a confirmation of its nephrotoxicity. Combined interpretation of the results with the data of biochemical study, biomarkers of renal damage and creatinine clearance suggests that, in the early period (1 to 3 days) after the poisoning, the changes registered are initially of afunctional nature, followed by development of changes in the structural elements of the nephron. Seven days after the poisoning, most of the revealed changes are normalized, but changes in GBM do not allow us to state that the consequences of poisoning are fully compensated.
[4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21]
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413625
References
- Wedin, G.P. Nephrotoxicity of anticholinesterases. In Clinical and Experimental Toxicology of Organophosphates and Carbomates; Ballantyne, B., Marrs, T.C., Eds.; Britterworth-Heinemann Ltd.: Oxford, UK, 1992; pp. 195–202. [CrossRef]
- Albright, R.K.; Kram, B.W.; White, R.P. Malathion exposure associated with acute renal failure. JAMA 1983, 250, 2469. [Google Scholar] [CrossRef]
- Kushnir, A.; Finkelstein, Y.; Raikhlin, B.; Taitelman, U. Multihospital study of severe acute organophosphate insecticide poisoning. Vet. Hum. Toxicol. 1988, 30, 366. [Google Scholar]
- Wedin, G.P.; Pennente, C.M.; Sachdev, S.S. Renal involvement in organophosphatc poisoning. JAMA 1984, 252, 1408. [Google Scholar] [CrossRef]
- Kaya, Y.; Bas, O.; Hanci, H.; Cankaya, S.; Nalbant, I.; Odaci, E.; Uydu, H.A.; Aslan, A. Acute renal involvement in organophosphate poisoning: Histological and immunochemical investigations. Ren. Fail. 2018, 40, 410–415. [Google Scholar] [CrossRef]
- Kerem, M.; Bedirli, N.; Guerbuez, N.; Ekinci, O.; Bedirli, A.; Akkaya, T.; Sakrak, O.; Pasaoglu, H. Effects of Acute Fenthion Toxicity on Liver and Kidney Function and Histology in Rats. Turk. J. Med. Sci. 2007, 37, 281–288. [Google Scholar]
- Boroushaki, M.T.; Arshadi, D.; Jalili-Rasti, H. Protective effect of pomegranate seed oil against acute toxicity of diazinon in rat kidney. Iran. J. Pharm. Res. 2013, 12, 821–827. [Google Scholar]
- Possamai, F.P.; Fortunato, J.J.; Feier, G.; Agostinho, F.; de Quevedo, J.L.; Filho, D.W.; Pizzol, F.D. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Environ. Toxicol. Pharmacol. 2007, 23, 198–204. [Google Scholar] [CrossRef]
- Al-Attar, A.M. Physiological and Histopathological Investigations on the Effects of α-Lipoic Acid in Rats Exposed to Malathion. J. Biomed. Biotechnol. 2010, 2010, 203503. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Mohamed, A.S.; Abdel-Wahhab, M.A. Chlorpyrifos-induced oxidative stress and histological changes in retinas and kidney in rats: Protective role of ascorbic acid and alpha tocopherol. Pestic. Biochem. Physiol. 2010, 98, 33–38. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, Y.; Li, S.; Qi, L.; Xu, W.; Wang, H.; Zhao, X.; Sun, C. Effect of quercetin against dichlorvos induced nephrotoxicity in rats. Exp. Toxicol. Pathol. 2014, 66, 211–218. [Google Scholar] [CrossRef]
- Satar, S.; Satar, D.; Mete, U.O.; Suchard, J.R.; Topal, M.; Kaya, M. Ultrastructural Effects of Acute Organophosphate Poisoning on Rat Kidney. Ren. Fail. 2005, 27, 623–627. [Google Scholar] [CrossRef]
- Eid, R.A. Apoptosis of rat renal cells by organophosphate pesticide, quinalphos: Ultrastructural study. Saudi J. Kidney Dis. Transpl. 2017, 28, 725–736. [Google Scholar] [PubMed]
- Fuentes-Delgado, V.H.; Martínez-Saldaña, M.C.; Rodríguez-Vázquez, M.L.; Reyes-Romero, M.A.; Reyes-Sánchez, J.L.; Jaramillo-Juárez, F. Renal damage induced by the pesticide methyl parathion in male Wistar rats. J. Toxicol. Env. Health Part A 2018, 81, 130–141. [Google Scholar] [CrossRef]
- Lee, F.Y.; Chen, W.K.; Lin, C.L.; Lai, C.Y.; Wu, Y.S.; Lin, I.C.; Kao, C.H. Organophosphate Poisoning and Subsequent Acute Kidney Injury Risk: A Nationwide Population-based Cohort Study. Medicine 2015, 94, 2107. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Munawar, K.; Nasrullah, A.; Haq, S.; Ghazanfar, H.; Sheikh, A.B.; Khan, A.Y. Renal failure due to organophosphate poisoning: A case report. Cureus 2017, 27, 523. [Google Scholar] [CrossRef]
- Cavari, Y.; Landau, D.; Sofer, S.; Leibson, T.; Lazar, I. Organophosphate poisoning-induced acute renal failure. Pediatr. Emerg. Care 2013, 29, 646–647. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghani, E.; Ghasemi, A.; Khoshbaten, A.; Asgari, A. Synaptosomal GABA uptake decreases in paraoxon-treated rat brain. Toxicology 2008, 244, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, M.O.; Sobolev, V.E.; Reshetkina, D.A.; Nagibovich, O.A. Toxic effect of organophosphate compounds on the kidneys. Bull. Russ. Mil. Med. Acad. 2020, 22, 199–205. (In Russian) [Google Scholar] [CrossRef]
- Saleh, A.M.; Vijayasarathy, C.; Masoud, L.; Kumar, L.; Shahin, A.; Kambal, A. Paraoxon induces apoptosis in EL4 cells via activation of mitochondrial pathways. Toxicol. Appl. Pharmacol. 2003, 190, 47–57. [Google Scholar] [CrossRef]
- Abbasnezhad, M.; Jafari, M.; Asgari, A.R.; Hajihoseini, R.; Hajigholamali, M.; Salehi, M.; Salimian, M. The study regarding effect of paraoxon on oxidative stress. J. Maz. Univ. Med. Sci. 2009, 19, 16–26. [Google Scholar]
- Jafari, M.; Salehi, M.; Asgari, A.; Ahmadi, S.; Abbasnezhad, M.; Hajihoosani, R.; Hajigholamali, M. Effects of paraoxon on serum biochemical parameters and oxidative stress induction in various tissues of Wistar and Norway rats. Env. Toxicol. Pharmacol. 2012, 34, 876–887. [Google Scholar] [CrossRef]
- Williams, R.I.; Pearson, J.E. Functional study of the renal effect of the anticholinesterase paraoxon. Arch. Int. Pharmacodyn. 1970, 184, 195–208. [Google Scholar]
- Abend, Y.; Goland, S.; Evron, E.; Sthoeger, Z.M.; Geltner, D. Acute renal failure complicating organophosphate intoxication. Ren. Fail. 1994, 16, 415–417. [Google Scholar] [CrossRef]
- Bentur, Y.; Nutenko, I.; Tsipiniuk, A.; Raikhlin-Eisenkraft, B.; Taitelman, U. Pharmacokinetics of obidoxime in organophosphate poisoning associated with renal failure. J. Toxicol. Clin. Toxicol. 1993, 31, 315–322. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Terpilovskij, M.A.; Shmurak, V.I.; Belinskaya, D.A.; Avdonin, P.V. Rat (Rattus norvegicus) as a research object in the model of acute intoxication with organophosphorous compound. 1. Biochemical aspects. J. Evol. Biochem. Physiol. 2019, 55, 112–123. [Google Scholar] [CrossRef]
- Sobolev, V.E.; Korf, E.A.; Goncharov, N.V. The Rat (Rattus norvegicus) as a Model Object for Acute Organophosphate Poisoning. 5. Morphofunctional Alterations in Kidneys. J. Evol. Biochem. Physiol. 2019, 55, 302–312. [Google Scholar] [CrossRef]
- Sobolev, V.E.; Jenkins, R.O.; Goncharov, N.V. Sulfated glycosaminoglycans in bladder tissue and urine of rats after acute exposure to paraoxon and cyclophosphamide. Exp. Toxicol. Pathol. 2017, 69, 339–347. [Google Scholar] [CrossRef]
