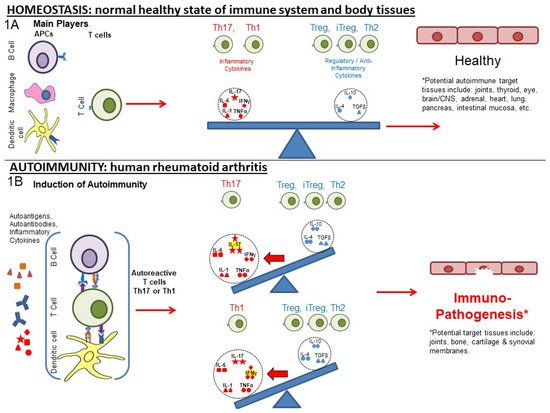
Rheumatoid arthritis (RA) and other autoimmune inflammatory diseases are examples of imbalances within the immune system (disrupted homeostasis) that arise from the effects of an accumulation of environmental and habitual insults over a lifetime, combined with genetic predispositions. The Ligand Epitope Antigen Presentation System (LEAPS) therapies are capable of inhibiting ongoing disease progression in animal models. Whereas DMARDs ablate or inhibit specific proinflammatory cytokines or cells and JAK inhibitors (jakinibs) inhibit the receptor activation cascade for expression of proinflammatory cytokines, the LEAPS therapeutic vaccines specifically modulate the ongoing antigen-specific, disease-driving, proinflammatory T memory cell responses. This decreases disease presentation and changes the cytokine conversation to decrease the expression of inflammatory cytokines while increasing the expression of regulatory cytokines.
- immunotherapy
- inflammatory
- anti-inflammatory
- cytokines
- rheumatoid arthritis
1. From Homeostasis to Autoimmunity
2. Post-Translational Modification and Its Role in Autoimmunity
3. Role of Cytokines, Cells, and Their Interplay in Disrupting Immune Homeostasis
4. Current and Older Therapeutic Approaches for RA
| Type | Target | ↓/↑ Modulation | Regulated Immune Component, If Known [References] | Generic and Product name, Regulatory Status | Ref. |
|---|---|---|---|---|---|
| Therapeutic Vaccines | Th1 | ↓ | IL-1, IL-17, IFN-γ, TNF-α [3,29,30] | CEL-4000 (preclinical) | [3,29,30] |
| ↑ | Treg (FOXP3+), IL-4, IL-10, TGF-β [3,29,30] | ||||
| Th17 | ↓ | TNF-α, IL-17, IL-6, MCP-1, IL-12p40 [27] | CEL-2000 (preclinical) | [27] | |
| ↑ | IL-12p70, IL-10 [27] | ||||
| DMARDs | TNF-α | ↓ | TNF-α [41] | Adalimumab (Humira®) | [41,42] |
| TNF-α | ↓ | TNF-α [43] | Etanercept (Enbrel®) | [43] | |
| IL-1Ra | ↓ | IL-1 [44] | Anakinra (Kineret®) | [44] | |
| IL-6R msR | ↓ | MCP-1 [42], IL-6 [45] | Tocilizumab (Actemra®) | [42,45] | |
| IL-17 | ↓ | MCP-1 [42], IL-17A [46] | Secukinumab (Cosentyx®) | [42,46] | |
| CD20 | ↓ | B cells as APCs: CD4+IFN-γ+, CD4+IL-17+ [47] | Rituximab (Rituxan®) | [47,48] | |
| Anti-CD6 | ↓ | IL-17 [49], IFN-γ [49,50], IL-6, TNF-α [50] | Itolizumab (Alzumab®) | [49,50,51] | |
| Agonistic Anti-CD137 | ↑ | IFN-γ [52,53], IDO [53] | Utomilumab | [52,53] | |
| Anti-CTLA4 | ↓ | IL-17, IFN-γ [54] | Abatacept (Orencia®) | [54,55,56] | |
| ↑ | IL-35, IFN-β [54] | ||||
| Anti-CD40 | ↓ | IL-6, RANKL [57], TNF-α, NF-κβ, IL-6, ICAM-1, VCAM-1, VEGF [58] | Bi 655064 | [57,58] | |
| CD24 | ↓ | TNF-α, IL-6, MCP-1(CCL2), IL-1β [59] NF-κβ [60] | [59,61,62] | ||
| Jakinibs | JAK3 > JAK1, JAK2 > TYK2 [63] | ↓ | Transcription: IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IFN-γ, > EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 > IL-12, IL-23, Type III IFNs [64] in vitro: IL-6 by B cells, [65] IL-2, IL-4, IL-7, IL-15, IL-21, IL-6, and IFN-γ in CD4+ T cells. IL-17 in Th17 cells polarized via IL-23. IL-21 and IL-22 in Th17 [66], IFN-α, IL-6, IFN-γ, IL-2, IL-15, IL-4, GM-CSF [64] MCP-1 [42] IL-17 in CD4+T cells from AS, PSA, RA, and HC [67] in vivo: IL-6 in human [68] |
Tofacitinib (Xeljanz®)FDA approved (2012) | [42,63,64,65,66,67,68,69,70,71] |
| ↑ | in vitro: IL-2 in Th1. IL-17, IL-2 in Th17 cells (polarized via TGF-β1, IL-6) [66] | ||||
| JAK3 > JAK1, TYK2, JAK2 [63] | ↓ | Transcription: IFN-α, IFN-β, IL-10, IL-22, IL-2, IL-4, IL-7, IL-9, IL-15, IL-2, IFN-γ > IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IL-12, IL-23, Type III IFNs, EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 in vitro: IL-4, IL-13, IFN-γ, TNF-α in PBMC after TCR stimulation, IL-4, IL-13, IFN-γ, TNF-α, IL-17A, GM-CSF in PBMC after IL-2 stimulation [72] |
Peficitinib (Smyraf®) Japan Approved (2019) | [63,72] | |
| JAK2, JAK1 > TYK2 > JAK3 [63] | ↓ | Transcription: IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IFN-γ > IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 > IL-12, IL-23, Type III IFNs, EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 [64] in vitro: IL-6 in MoDCs, IFN-α secreted pDCs [65] MCP-1 [42] IL-17 in CD4+ T cells (AS, PSA, RA, and HC) [67] |
Baricitnib (Olumiant®) FDA approved (2018) | [42,63,64,65,67,69,70] | |
| JAK2, JAK1 > TYK2 > JAK3 [63] | ↓ | Transcription: IFN-γ, EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 > IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IL-12, IL-23, Type III IFNs > IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 in vitro: IL-10, IFN-γ, IL-6, TNF-α, IL-13 [73] IL-17 in CD4+ (AS, PSA, RA, and HC) [67] in vivo: IFN-γ, IL-12p70, IL-6, G-CSF, IL-10, TNF-α [73] |
Ruxolitinib (Jakafi®) FDA approved (2011) (myelofibrosis) | [63,67,69,73,74] | |
| ↑ | in vitro: IL-2 [74] | ||||
| JAK1 > JAK2 > TYK2 > JAK3 [63] | ↓ | Transcription: IL-6, IL-11, IL-13, IL-25, IL-27, IL-31 > IFN-α, IFN-β, IL-10, IL-22 > IFN-γ, > IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 > EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5, IL-12, IL-23, Type III IFNs [64] in vitro: IL-2, IL-4, IFN-αB2, IFN-γ [71] IFN-α, IL-6, IFN-γ, IL-2, IL-15, IL-4 [64] ex vivo: IL-6, GM-CSF [64] in vivo: IFN-γ, IL-6, IL-1β, RANKL, MMP-3, MMP-13, IP10, XCL1, MCP-1, MIP-1b, MCP-3, MCP-5, M-CSF1, MDC, SCF, KC/GRO, IL-1α [71] SAA, IL-6, IL-1β, GM-CSF, TNF-RI, Resistin, TNF-α, MMP-3, YKL40, VEGF, MMP-1, IL-12, IL-2, IFN-γ, IL-13, IL-5, IL-21, IL-23, IL-17A, IL-7, IL-10, CXCL10, CXCL13, MCP-1, VCAM-1, MIP-1a [75] |
Filgotinib (Jyseleca®) EMA & Japan approved (2020) | [63,64,69,71,75] | |
| JAK1 > JAK2 > JAK3 > TYK2 [63] | ↓ | Transcription: IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IFN-γ EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 > IL-12, IL-23, Type III IFNs [64] in vitro: IFN-α, IL-6, IFN-γ, IL-2, IL-4, IL-15, G-CSF [64] |
Upadacitinib (Rinvoq®) FDA approved (2019) | [63,64,70] | |
| JAK2 > JAK1 > TYK2 > JAK3 [76] | ↓ | See main text in vitro: VCAM-1, IL-6 [74] |
Fedratinib (Inrebic®) (2019) (myelofibrosis) | [63,74,77,78,79] | |
| ↑ | in vitro: IL-2 [74] |
5. Grouping of the Therapeutic Approaches
6. Comparisons of LEAPS, Monoablative, and Jakinib Therapies
LEAPS peptide therapeutic vaccines are designed to have an immunomodulatory effect on the T cells driving the disease, as illustrated in Figure 3A,B. In so doing, the LEAPS peptides affect the entire cytokine conversation, increasing the expression of some and decreasing other cytokines to return immunobalance, rather than acting on a single cytokine [3,27,29,30,31].
The monoablative therapies (shown in Figure 3C,D) use a neutralizing monoclonal antibody or receptor-antagonist to specifically block the action of one of the disease-associated cytokines after secretion and not its synthesis. The targets for these therapies include IL-1β, IL-6, IL-17A, IL-23, IL-12, and TNF-α and, under certain circumstances, IFN-γ. These monoablation therapies only indirectly affect other pro-inflammatory cytokines and do not upregulate anti-inflammatory cytokines to rebalance the cytokine conversation.

The third group (III) of therapies (Table 1, Figure 3E–G) are the jakinibs, which act by inhibiting specific receptor-associated JAK/STAT tyrosine kinases, ultimately inhibiting the synthesis and secretion of multiple cytokines (multi-ablative therapy) that are activated by the specific JAK cascade. The jakinibs are small-molecule (~300Da) inhibitors acting on the Janus kinases JAK1, JAK2, JAK3 or TYK2 and have the major advantage of being taken orally [63]. The JAK enzymes most often work in pairs as homo- or heterocomplexes activating STAT molecules to create transcriptional activators and promote the expression of groups of cytokines and other genes. JAK activation or inhibition also influences the expression of different cell surface receptors, including CD4, CD80 and CD86 on T, B and other cells and their associated immune responses [65]. The expression of some JAK enzymes is more restricted to certain cell types than others, such as JAK3 for immune system cells such as B, T, and NK cells. This is a therapeutic advantage.
As can be seen in Table 1, there are at least five different jakinibs approved for RA treatment in the USA, Europe, or Japan and several others are under investigation, each unique in terms of the molecule’s binding preference for its particular cognate JAK or JAK-associated molecule. Different manifestations of treatment occur depending on the relative selectivity of binding and whether it is reversible or irreversible. The representative jakinibs are shown as different-colored (red, blue and orange) hexagonal shapes in Figure 3 for the three examples of jakinibs that downregulate inflammatory and anti-inflammatory cytokines. Jakinib A (Figure 3E red hexagon) has activity which is JAK 3>1 and downregulates the expression of IL-6, IL-17, IFN-γ, TNF-α, IL-4, and IL-10. Jakinib B (Figure 3F blue hexagon) is a JAK 2>1 inhibitor downregulating IL-6, IL-17, IFN-γ, TNF-α, and IL-10. Jakinib C (Figure 3G orange hexagon) is a JAK 1>2 inhibitor downregulating IL-1, IL-6, IL-17, IFN-γ, TNF-α, IL-4, and IL-10. It should be noted that a jakinib specific for only JAK2 cannot be used since the inhibition of JAK2/STAT is associated with lethality early in life [63,77].
Although TNF-α, IL-1, and IL-17 are major targets for monoablation therapy, they are not directly affected by the jakinibs. However, they may be indirectly affected by the inhibition of expression of other cytokines that are supposedly not involved in the JAK signaling pathway; for example, IL-17 is affected indirectly by several of these jakinibs [66,67,77]. Similarly, an indirect effect of jakinibs may also promote the expression of some anti-inflammatory cytokines. The combination therapy targeting several cytokines, as possibly seen for the JAK inhibitors, may be effective, although this is still being debated and new clinical studies will be needed [2,99,111,112].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10010044
