2.1. Gross Anatomyof the Male Reproductive Tract
Testicular growth was slow from 0 to 8 months old, with a sharp increase occurring from 9 months, and testicular biometric parameters being highly correlated to age and body weight of the animals [
14]. A pair of oval, pinkish-brown testes were located within scrotal pouches intra-abdominally [
15]. Externally, the testes were seen as two subcutaneous lumps on either side of the inner hind legs. The mean testicular volume was 5.39 ± 0.32 cm
3, while the gonadosomatic index was 0.48 ± 0.02% [
15]. Some authors found that the mean testis weight for
D. leporina was 4.1 ± 0.6 g [
16].
Table 1 lists the mean measurements of the testes. Menezes et al. [
17] further stated that the external part of the genitals was hidden ventrally within a skin sac, and the scrotal sacs were not clearly demarcated.
Table 1. Gross measurements of the male reproductive tract of the agouti (
D. leporina), according to Mollineau et al. [
15].
| Component |
Mean Length (cm) |
Mean Diameter or Width (cm) |
Mean Weight (g) |
| Testes |
3.67 ± 0.12 |
1.67 ± 0.04 |
5.03 ± 0.52 |
| Vas deferens |
10.98 ± 0.40 |
0.14 ± 0.01 |
- |
| Seminal vesicles |
4.76 ± 0.15 |
1.03 ± 0.04 |
1.28 ± 0.16 |
| Coagulating glands |
3.10 ± 0.22 |
1.74 ± 0.12 |
1.23 ± 0.23 |
| Prostate glands |
3.50 ± 0.12 |
1.10 ± 0.02 |
0.99 ± 0.41 |
| Bulbourethral glands |
1.47 ± 0.01 |
1.65 ± 0.11 |
1.27 ± 0.16 |
| Penis |
9.90 ± 0.43 |
1.60 ± 0.17 |
4.72 ± 0.25 |
| Glans penis |
3.18 ± 0.22 |
0.80 ± 0.18 |
1.80 ± 0.04 |
| Corpus penis |
6.72 ± 0.43 |
0.76 ± 0.03 |
2.94 ± 0.25 |
The epididymis, which ran the entire length of the testis, had a coiled head enclosed by fat, with an increase in coiling of the body towards the cauda epididymis. The paired vas deferens were creamish-white in color and thick-walled, and terminated in the urethra. The diameter of the vas deferens expanded to form the ampulla (mean diameter 0.25 cm) close to the urethral end [
15].
The accessory sex glands of the agouti included the seminal vesicles, the coagulating glands, the prostate gland and the bulbourethral glands [
15,
17]. The seminal vesicles, which were branched, tightly coiled and pale yellowish-cream in color, were found to be the largest of the accessory sex glands. They were located at the dorsal end of the vas deferens and attached via a short stem, which continued into a main branch from which a number of lobes originated [
15,
18]. The coagulating glands, found on either side of the urethra, were irregularly shaped structures with a purple, light brown gel-like appearance, composed of lobes which emanated from the base and ran the entire length of the coagulating glands [
15,
18]. They were made up of many tubules within the main duct of the longitudinal part of the seminal vesicles, and were partially covered by the prostate gland, with tubules running caudally to the urethra and draining into the prostate [
18].
The prostate glands were found dorsal to the coagulating glands, and had a similar structure to the seminal vesicles. These paired lobulated structures were creamish-white in color, and had forty to fifty lobes which were slightly spongy, and mostly looped backwards [
15]. This description was inconsistent with that of Menezes et al. [
18], who described one prostate gland being divided into two equal lobes; a ventral lobe (located laterally to the urethra and divided into a dorsal and a ventral area) and a dorsal lobe (also divided and found on either side of the urethra).
The paired bulbourethral glands were bean-shaped and located ventro-lateral to the rectum, and dorsal to the pubic symphysis [
15]. This disagreed with Menezes et al. [
18] who found that the paired bulbourethral glands were located dorso-lateral to the rectum and dorso-cranial to the anal sphincter muscles. The glands were latero-medially compressed, covered dorsally by subcutaneous tissue. They contained one duct that opened into the urethra, before the formation of the penis bulb [
15].
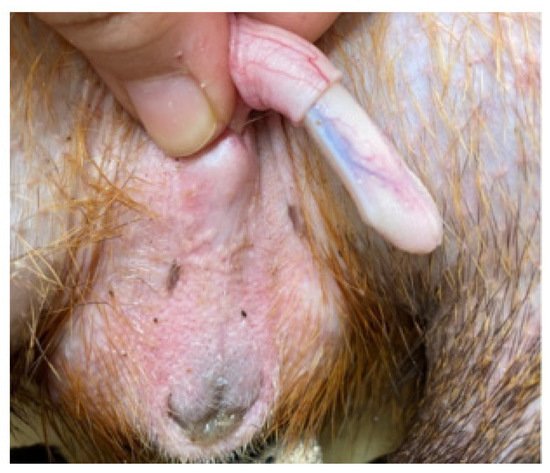
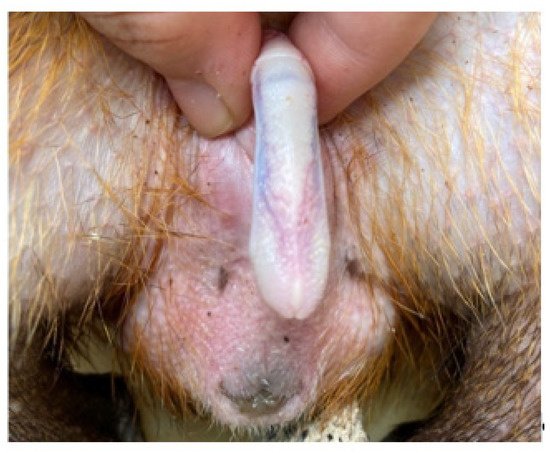
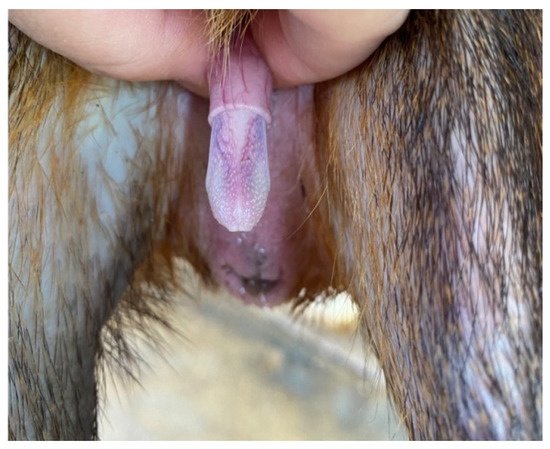
The penis, situated subcutaneously on the ventral side of the pubic symphysis, was cylindrical, laterally compressed, caudally directed and U-shaped [
15,
17]. The penis was made up of two parts; the glans (glans penis; covered by the prepuce) and the body (corpus penis), which had a distinct junction located just before the bend in the U-shaped penis. The glans penis was covered by penile spines that were raised and directed towards the junction (see
Figure 1,
Figure 2 and
Figure 3). Within the dorsal part of the glans penis was the os penis (2.5 cm in length) [
15]. An intromittent sac opened into a slit ventral to the urethral opening, in which two keratinaceous styles were located. The cranial ends of these keratinaceous styles were attached to the caudal end of the intromittent sac and had a mean length of 1.20 ± 0.03 cm. A lateral penile cartilage was found on either side of the glans. The dorsal edge was attached to the lateral side of the glans penis, while its ventral side was free [
15]. These descriptions were similar to those of Menezes et al. [
17], who also found the presence of the dartos tunic, external and internal spermatic fasciae and cremaster muscle, upon removal of the skin.
Figure 1. Penis showing lateral cartilage.
Figure 2. Dorsal view of penis.
Figure 3. Penile spines visible.
Some of these anatomical features worked together in order for erection to take place. Four stages of erection were identified: the penis protruded out of the preputial orifice during stage 1; the lateral penile cartilages were raised vertically and spread laterally during stage 2; stage 3 involved the formation of a penile flower and eversion of the intromittent sac; stage 4 was the completion of erection and ejaculation [
19].
2.2. Histology of the Male Reproductive Tract
Costa et al. [
16] suggested that agoutis were good candidates for increasing their reproductive performance in livestock programs. They had high spermatogenic efficiency and the same seminiferous epithelium cycle pattern and relative stage frequencies as the
Agouti paca.
Eleven spermatozoa morphologies were reported. The most abundant spermatozoa were normal, while ten had defects, such as a piriform head, micro-cephalic head, retained cytoplasmic droplet, bent neck, double tail, and coiled tail. The spermatozoon was found to have an oval-shaped head, a streamlined mid piece and a thin, tapered tail [
20,
21]. The lateral penile cartilage consisted of perichondrium, chondroblasts, chondrocytes, intercellular substance, isogenous groups or nest cells, blood vessels, nerves and glands. The peripheral layer consisted of collagenous fibers and fibroblasts, while the second layer (cellular layer) consisted of mesenchymal cells [
15]. The corpora cavernosa was made up of erectile tissue covered by a thick layer of dense connective tissue (tunica albuginea). The spongy body of the penis was comprised of erectile tissue around the urethra, lined with endothelium and surrounded by a layer of dense connective tissue (the white tunic of the spongy body) [
17].
The seminal vesicles were branched and the mucosa, which was thrown into folds, was lined by a pseudo-stratified columnar epithelium. It consisted of a lamina muscularis mucosa, a tunica muscularis, and a tunica serosa [
22]. These findings were also consistent with those of Menezes et al. [
18]. There was a significant relationship between the length of the seminal vesicles and the length of the epithelium folds.
Table 2 shows the microscopic dimensions of the seminal vesicles.
Table 2. The mean microscopic measurements of some of the components of the male reproductive tract of the agouti (
D. leporina), according to Mollineau et al. [
22].
| |
Mean Diameter of Lumen (µm) |
Mean Width of Tunica Mucosa (µm) |
Mean Width of Tunica Muscularis (µm) |
Mean Width of Tunica Serosa (µm) |
| Seminal vesicle |
883.6 ± 76.83 |
24.1 ± 0.92 |
233.1 ± 26.40 |
38.3 ± 4.26 |
| Coagulating glands |
488.3 ± 41.96 |
15.0 ± 1.25 |
84.5 ± 6.86 |
24.2 ± 1.84 |
| Prostate |
995.2 ± 55.70 |
13.9 ± 1.16 |
34.2 ± 3.22 |
39.6 ± 3.73 |
The mucosa of the coagulating glands was leaf-like and contained tubulo-alveolar glands. The epithelium had apical blebs and cilia on the surface and consisted mainly of pseudo-stratified columnar cells (consistent with the findings of Menezes et al. [
18]), with some simple columnar cells. Beneath the epithelium was the lamina propria; however, no distinct lamina muscularis mucosa or tunica submucosa was identifiable. The tunica muscularis contained fibroblasts dispersed between the smooth muscle fibers, and the tunica serosa contained areolar connective tissue in which there were blood vessels [
22].
The epithelium of the prostate consisted of pseudo-stratified columnar cells and was folded, thus creating a large lumen [
18,
22]. The mucosa contained tubular and tubulo-alveolar glands. Apart from the epithelial layer, the other layers included the lamina propria, tunica muscularis, and tunica serosa. There was no observable separation between the lamina muscularis mucosa and the tunica submucosa.
The bulbourethral gland consisted of densely packed convoluted tubules with mucous secretory units, and poorly stained cytoplasm and vacuoles [
18,
22]. The gland was covered by a thick, stratified skeletal tissue layer, and surrounded by a thin capsule of conjunctive tissue [
18]. The epithelial lining was made up of simple columnar epithelial cells with oval nuclei, which were located near the basement membrane. These cells were surrounded by a lamina propria [
22].
Spermatogonial cells (Type A-pale, Type A-dark, intermediate, and Type B) were found in the germinal epithelium of the seminiferous tubules in pre-pubescence, pre-puberty, pubescence and sexually mature agoutis [
23,
24]. Similar classifications were also used by Assis-Neto et al. [
24] in the analysis of spermatogenesis, where they found that birth to five months old was pre-pubescent; six to eight months old was the transition to puberty; puberty occurred at nine to ten months old, and post-puberty from twelve to fourteen months old.
Some primary spermatocytes were found in the seminiferous cords of pre-pubescent agoutis (in prophase and its sub-phases) [
23]. Spermatocytes, in the pachytene phase, were abundant among primary spermatocytes. Sertoli cells were found in all age groups; they exhibited nuclear membrane invaginations and lipid inclusions in the cytoplasm in the pre-pubertal phase. Leydig cells were also discovered in all age groups, but were most abundant in the pubescent and mature groups, and displayed higher metabolic activity during puberty. Spermatozoa were morphologically fully formed at pre-puberty [
23].
Costa et al. [
16] characterized eight stages of the seminiferous epithelium cycle. They found that only one spermatid generation occurred in stage one and the spermatids, which formed several layers in the upper part of the seminiferous epithelium, had round nuclei. Pre-leptotene spermatocytes, pachytene spermatocytes and Type A spermatogonia were also seen. In stage two, the spermatid nuclei started to elongate, and the chromatin was more condensed than previously. Pre-leptotene spermatocytes were transitioning to leptotene and pachytene spermatocytes. Type A spermatogonia were still present. In stage three, the spermatids formed bundles, and the primary spermatocytes were in leptotene and diplotene. Type A spermatogonia were still present. In stage four, meiotic figures of the first and second divisions were observed. Secondary spermatocytes, zygotene and diplotene spermatocytes were also noted. The spermatid bundles were located within Sertoli cells, and Type A spermatogonia were still present. In stage five, two generations of spermatids were seen (newly formed round and elongated spermatids). The spermatid bundles were more packed, and some were located deep within the epithelium. Early pachytene spermatocytes were the predominant cell type located between spermatids and the basal lamina. Type A spermatogonia nuclei were seen. In stage six, the spermatid bundles were close to the seminiferous tubule lumen. Pachytene spermatocyte nuclei were further from the basal lamina and intermediate spermatogonia were seen. Type A spermatogonia were occasionally present. In stage seven the spermatid bundles dissociated, and spermatid nuclei were close to the tubule lumen; small residual bodies were also present. Type B spermatogonia, with round to ovoid nuclei and large amounts of heterochromatin, were seen, while Type A spermatogonia were intermittently found. In addition, also present were pachytene spermatocytes and round and elongated spermatids. In stage eight, the elongated spermatids (with large residual bodies below) were about to be released at the luminal portion of the seminiferous tubule, and pre-leptotene spermatocytes were located close to the basal lamina.
Costa et al. [
16] also reported on the stereology of the Leydig cells: the nuclear diameter was 8.2 ± 0.1 µm; cell volume was 1230 ± 70 µm
3 (nucleus volume: 280 ± 12 µm
3; cytoplasm volume: 950 ± 60 µm
3); cell number per testis was 70 ± 19 × 10
6; and cell number per gram of testis was 18 ± 3 × 10
6. In addition, they found that for Sertoli cells, the cell number per testis was 204 ± 30 × 10
6, and cell number per gram of testis was 57 ± 6 × 10
6.
The epididymis had a pseudo-stratified, columnar, stereociliated epithelium. Younger, pre-pubescent, agoutis had more clean cells, and older males had more apical cells. Based on morphology, other cell types such as principal, basal and halogen cells were identified. Peri-tubular myoid cells, present in the vas deferens and epididymis, were found to be more abundant after puberty. The vas deferens was composed of muscular layers (two layers in pre-pubescent and pre-pubertal animals and three layers in pubertal and adult animals) and mucosa (pseudo-stratified epithelium) [
25].
2.3. Reproductive Technologies
Mollineau et al. [
26] pioneered the use of electro-ejaculation for collecting spermatozoa from anaesthetized male agoutis, using a lubricated electro-ejaculator probe (12.7 cm long; 1 cm in diameter) inserted 8 cm in the rectum. Anaesthesia was performed using ketamine, intramuscularly, five minutes before electro-ejaculation was performed. However, this protocol was improved by the inclusion of lower dosages of xylazine in combination with ketamine, since xylazine at a dosage of 40 mg/kg resulted in 75% of ejaculate samples containing spermatozoa [
27]. To conduct the electro-ejaculation stimuli sequence, six volts were applied for a five second period (on period), followed by a five second rest period (off time). This sequence was repeated using voltages increasing incrementally by one volt, until a maximum of twelve volts was attained. Upon reaching twelve volts, the sequence was repeated until the agouti ejaculated, or until ten minutes had elapsed [
26]. This resulted in spermatozoa being present in only 30% of the ejaculate samples. It was concluded that the maximum electro-ejaculation time should be six minutes, with off periods of three to four seconds. The utilized electro-ejaculation technique was able to yield an average spermatozoa concentration of 106.7 ± 31.1 × 10
6 spermatozoa/mL, while the highest spermatozoa concentration yielded by Mollineau et al. [
27] was 431 ± 180 × 10
6 spermatozoa/mL.
In contrast, Martinez et al. [
28] were able to obtain spermatozoa from all four electro-ejaculated
D. azarae males. However, a different anesthetic protocol was used whereby they pre-anaesthetized the animals with azaperone (4 mg/kg) and meperidine (4 mg/kg), intramuscularly. Anesthesia was then induced ten minutes later, using xylazine hydrochloride (0.4 mg/kg) and ketamine hydrochloride (20 mg/kg) intramuscularly, followed five minutes later by a lumbosacral application of lidocaine (5 mg/kg). They also utilized another type of electro-ejaculator for wild animals, and the stimuli sequence was different. They conducted a series of four sets of twenty stimuli, starting at two volts, followed by four volts, then six volts and eight volts, with an on period of three seconds and two-minute intervals between each series. Ejaculation (100%) was obtained using six volts, unlike the average ejaculation voltage of 9.33 ± 0.69 V, obtained by Mollineau et al. [
26].
Serial and continuous stimuli and electro-ejaculation using ring and longitudinal electrodes, emitting sine waves and square waves, respectively, were evaluated for efficient semen collection [
29]. The agoutis were anaesthetized using ketamine (35 mg/kg) and xylazine (5 mg/kg) intramuscularly. It was concluded that ring electrodes with a serial stimuli protocol improved the efficiency of semen collection via electro-ejaculation as 57% of ejaculate samples contained spermatozoa, as compared with 41.33% and 40.8% [
27] and 30% [
26].
Anesthetic protocols that used xylazine (2.5 mg/kg, intramuscularly), ketamine (20 mg/kg, intramuscularly) and a lidocaine hydrochloride lumbosacral epidural (5 mg/kg), or dexmedetomidine (25 µg/kg, intramuscularly) and ketamine (35 mg/kg, intramuscularly), not only facilitated spermatozoa collection, but also provided better analgesia, as reflected by the vital signs recorded [
30].
Retrograde epididymal washing was another means of recovering spermatozoa [
21,
31,
32,
33]. The left testis obtained from castration was utilized [
21]. An anesthetic protocol of 5 mg/kg pethidine hydrochloride, intramuscularly, was used as the pre-anesthetic approximately ten minutes before induction, and maintenance was performed using a combination of 35 mg/kg ketamine hydrochloride and 1 mg/kg xylazine hydrochloride, intramuscularly. The epididymal spermatozoa were then collected after separating the testis-epididymis complex and rinsing the cauda epididymis with 0.2 mL of physiological saline (room temperature). The results from this experiment were satisfactory, as viable spermatozoa was collected from all test subjects, with an average spermatozoa concentration of 748 ± 418.66 × 10
6 spermatozoa/mL [
21].
Agoutis were euthanized to obtain both testes and a similar procedure, as described above, was used for retrograde epididymal washing [
31]. Flushing media (0.5 mL) was used, which was either powdered coconut water (ACP-109c) or Tris extenders. It was found that both solutions were effective, with powdered coconut water (ACP-109c) yielding 300 ± 2 µL and 1.1 ± 0.3 × 10
9 spermatozoa/mL, and Tris extenders yielding 200 ± 1 µL and 1.1 ± 0.2 × 10
9 spermatozoa/mL. Similarly, Silva et al. [
32] euthanized agoutis and performed retrograde epididymal washing using coconut water (ACP-109c), as described by Silva et al. [
31], and yielded 300 ± 2 µL and 1.4 ± 0.3 × 10
9 spermatozoa/mL. The same method used by Silva et al. [
31,
32] was later utilized by Castelo et al. [
33] in euthanized
D. leporine, and yielded a volume of 1650 ± 220 µL and 1.04 ± 0.2 × 10
9 spermatozoa/mL.
Whole cow’s milk at an ultra-high temperature, unpasteurized coconut water, and pasteurized coconut water, were evaluated for their use as semen extenders [
34]. The extenders were frozen in plastic bottles (25 mL of extender/bottle), which were subsequently cooled to 5 °C. In the first experiment, the ejaculate samples were diluted with the respective semen extender to 50, 100, 150 and 200 × 10
6 spermatozoa/mL by slowly adding half of the required volume of semen extender to the ejaculate, and adding the other half twenty minutes later. The samples were filled into 0.25 mL microtubes and refrigerated at 5 °C. The second experiment followed this same method, except for the fact that the sample size per treatment was smaller, and the samples were stored in frozen pellets in liquid nitrogen (−195 °C). Subsequently, samples were thawed in water baths of different temperatures (30, 40, 50, 60 and 70 °C for 20, 30, 40 or 50 s. It was concluded that samples extended with ultra-high temperature whole cow’s milk (100 × 10
6 spermatozoa/mL) gave the best result in experiment one, as it had the slowest rate of deterioration and the highest means for forward progressive motility % (FPM%) of 59.5 ± 7.75 after 1 day, and 22.0 ± 6.24 after 5 days. For experiment two, the samples that were thawed at 30 °C for 20 s had the highest means for FPM% (12.18 ± 1.33%) and a 85% rate of deterioration [
34].
The recovery and cryopreservation of epididymal sperm using powdered coconut water (ACP-109c) and Tris extenders was also performed by other authors [
31]. The initial centrifuged samples were extended with either diluent or egg yolk (20%) and stored at 27 °C, and later cooled to 4 °C. Semen was then added to either the powdered coconut water (ACP-109c) or the Tris extenders with egg yolk and 12% glycerol, to result with 6% glycerol in the final extender. The samples were filled into 0.25 mL straws and stored in liquid nitrogen. One week later, after thawing at 37 °C for one minute, it was concluded that powdered coconut water (ACP-109c) was a better extender than Tris extenders, as it had 26.5 ± 2.6% motile sperm with 2.6 ± 0.2 vigor, compared with 9.7 ± 2.6% motile sperm with 1.2 ± 0.3 vigor.
Epididymal sperm could be cryopreserved in either 0.25 mL or 0.50 mL straws. It was recommended that thawing should be done at 37 °C for one minute, as sperm motility was found to be 26.5 ± 2.6% (0.25 mL straw) and 18.4 ± 3% (0.50 mL straw) when thawed at this temperature [
32]. Four cryoprotectants (glycerol [3% and 6%], ethylene glycol, dimethylformamide and dimethylsulfoxide [3% and 6%]) were evaluated for their use in the cryopreservation of epididymal sperm [
33]. It was concluded that glycerol (3% and 6%) and dimethylsulfoxide (3%) could be used as cryoprotectants, with their post-thawing sperm motility values of 39.5% (glycerol) and 29.5% (dimethylsulfoxide) being better than that achieved by Silva et al. [
31].