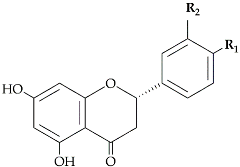
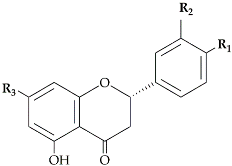
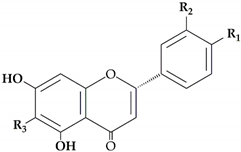
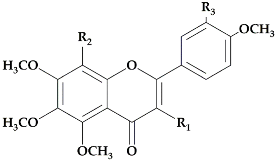
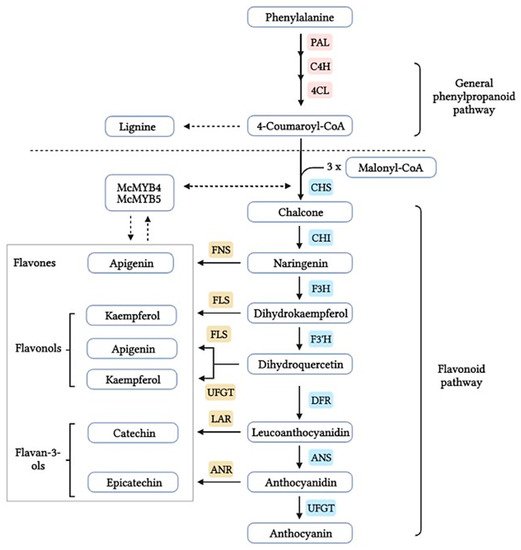
The flavonoid pathway is preceded by the general phenylpropanoid pathway, in which three enzymes are involved in the conversion of the amino acid phenylalanine to 4-coumaroyl-CoA. the first enzyme, phenylalanine ammonia lyase (PAL:EC 4.3.1.24) catalyzes the conversion of the amino acid phenylalanine to
trans-cinnamic acid, with the release of ammonia (NH3), then the two other enzymes (the enzyme cinnamate 4-hydroxylase (C4H:EC 1.14.14.91), followed by 4-coumarate-CoA ligase (4CL:EC 6.2.1.12)), catalyze the reaction that leads to obtain 4-coumaroyl-CoA, which is an important precursor in the flavonoid pathway [
12,
13]. The biosynthesis of flavonoids is originated from the phenylpropanoids pathway and initiated by two precursors named malonyl-CoA and
p-coumaroyl-CoA (
Figure 1). After the condensation of three acetate units from malonyl-CoA with one molecule of
p-coumaroyl-CoA, naringenin chalcones are formed. Naringenin chalcone, a major pigment of many flowers, leaves and fruits, is converted to naringenin by chalcone isomerase (CHI) or non-enzymatically in vitro [
14,
15]. This reaction catalyzed by chalcone synthase (CHS:EC 2.3.1.74) is thought to be the key regulatory step in the synthesis of flavonoids. It catalyzes the stereospecific isomerization of chalcones to their corresponding (2S)-flavanones via an acid base catalysis mechanism; the unstable chalcone form is normally isomerized by the enzyme chalcone isomerase (CHI:EC 5.5.1.6) to form the structural precursors for a broad range of flavonoids, such as flavonols, flavanones, anthocyanin glycosides and other derived compounds (
Figure 1).
(2
S)-Flavanones are converted stereospecifically to the respective (2
R,3
R)-dihydroflavonols (DHFs) by flavanone 3-hydroxylase (F3H:EC 1.14.11.9) in this case to dihydrokaempferol, then converted by the enzyme flavonoid 3′-hydroxylase (F3′H:EC 1.14.14.82) to dihydroquercetin. In
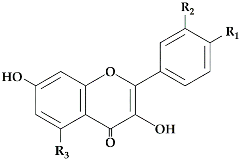
Citrus species, the two dihydroflavanols are then converted into flavonols under the action of flavonols synthetase (FLS:EC 1.14.20.6). UDP-glucose flavanone-7-
O-glucosyltransferase (UFGT:EC 2.4.1.115) and UDP-rhamnose flavanone glucoside rhamnosyltransferase (UFGRT) catalyze the conversion of the flavanone aglycones into their glucosides and rhamnoglucosides [
16]. Dihydroflavonol-4-reductase (DFR:EC 1.1.1.219) is a key enzyme required for both anthocyanin biosynthesis and proanthocyanidin accumulation and is involved in the conversion of dihydroflavonols to leucoanthocyanidins [
17].
3. Flavonoid Composition in Different Parts of Citrus Fruits
It has been found that
Citrus flavonoids are present in almost all the parts of
Citrus fruits in different species. Several studies showed that peels are the main sources of polyphenols in
Citrus fruits [
19]. Seed and peel extracts from numerous
Citrus species have proved to be an important source of flavonoids, such as polymethoxyflavones (PMFs), flavanones, and glycosylated flavanones [
20]. More than 5000 flavonoid molecules have been identified [
21].
Citrus juice is also a major source of flavanone glycosides. Moreover, the contents and types of flavonoids vary among different
Citrus species and fruit parts [
21]. Flavanones are the most important flavonoids in Citrus species. Hesperedin and naringenin are the predominant flavanones in Citrus fruits [
16]. They can be found in all plant parts: stem, branches, bark, flowers, leaves, roots, rhizomes, seeds, fruits, peels, etc. Flavones are the second major group of flavonoids in
Citrus, with flavonols in third place because their content is much lower than the other flavonoids [
17,
18]. The content of these components depends upon the age of the plant; most
Citrus species accumulate substantial quantities of flavonoids during organ development. Another study showed that flavonoids content might be affected by the maturity of the fruit, the post-harvesting treatments and the extracting processes [
22]. Juice preparation and the processing of fresh
Citrus fruits may decrease flavonoids content by 50% due to their water washing methods [
23,
24], or by elimination of the richest parts of the fruit [
25].
In this review, we gather some relevant data for the most abundant flavonoid component in sweet orange (
C. sinensis), sour orange (
C. aurantium), mandarin orange (
C. reticulata), clementine (
C. Clementina), lemon (
C. limon), and grapefruit (
C. Pardisi) juices and peel. It should be noted that
Citrus peel exhibit higher contents of flavanones than other parts of the fruit [
11]. Depending on their varieties, grapefruit and sour orange are very rich in naringin. Other
Citrus species such as sweet orange, and lemon have low quantities of naringin (
Table 3). Pupin et al. [
26] studied the composition of flavanone glycosides in various orange juice varieties and concluded that narirutin and hesperidin are the most abundant compounds. Hesperidin is present in high levels in clementine, sweet orange, mandarin orange and lemon (39.9–20.5 mg/100 mL juice), while naringin (23 mg/100 mL juice) and narirutin (7.6 mg/100 mL juice), glycosides of naringenin, are especially more abundant in grapefruit (
Table 3) [
27]. In lemon, eriocitrin is present in high level (16.17 mg/100 mL juice) [
26]. Didymin is a typical flavonoid glycoside also known as neoponcirin used in Asian countries as a dietary antioxidant [
28]. This flavonoid is mainly present in orange, mandarin and grapefruit (1.89–0.30 mg/100 mL juice) (
Table 3).
The most abundant flavanones in
Citrus peel are naringin andhesperidin. These flavanones are all glycosylated either by rutinose (6-O-
α-
l-rhamnosyl-
d-glucose) or by neohesperidose (2-O-
α-
l-rhamnosyl-
d-glucose) linked in position 7 [
28]. Hesperidin (0.002 to 9.42 mg/g peel dry basis) [
29,
30] is the main flavanone in all lemon cultivars while levels of diosmin and eriocitrin are the lowest [
31]. Mandarin peel is rich in hesperidin (3.95 to 80.90 mg/g peel dry basis) [
32,
33], narirutin (7.66 to 15.3 mg/g peel dry basis) [
22,
34], and naringin (0.54 to 0.65 mg/g peel dry basis) [
32,
33]. Naringin is the most abundant flavonoid in grapefruit and bitter orange peel, conferring a characteristic bitter taste (10.26 to 14.40 mg/g peel dry basis) [
29,
35].
Citrus peels contain also polymethoxyl flavones, such as sinensetin (0.08 to 0.29 mg/g dry bassis), nobiletin (0.2 to 14.05 mg/g dry basis), tangeretin (0.16 to 7.99 mg/g dry basis) and heptamethoxyflavone [
26,
36,
37,
38]. The glycosylated flavones are present in small quantities in
Citrus peel, such as diosmin, rhoifoline, isorhoifoline, luteolin. Other flavonoids are present in very small quantities in citrus peel, such as flavonols (quercetin, rutin, myricetin, and kaempferol) [
39].
Several studies have shown that the extracts of seeds and leaves of
Citrus contain high quantities of phenolic compounds, such as flavonoids [
40,
41]. Naringin is the most abundant flavonoid in grapefruit seeds (0.2 mg/g seeds) [
41]. The flavonoid content in
Citrus peel is much higher than in the seeds. They appear in plants and foods mostly as glycosides [
42].
Table 3. Flavonoid composition of some
Citrus juices [
4,
43,
44].
| |
Flavanones |
Flavones |
Total Flavonoids Content |
| |
Hesperidin |
Narirutin |
Naringin |
Didymin |
Eriocitrin |
Diosmin |
6,8-di-C-Glu-Diosmetin |
6,8-di-C-Glu-Apigenin |
Sinensitin |
| Orange |
28.6 |
5.2 |
- |
1.89 |
0.31 |
0.09 |
0.35 |
5.72 |
0.37 |
18.34 |
| Sour orange |
- |
- |
1.97 |
- |
- |
0.15 |
- |
- |
- |
- |
| Mandarin orange |
24.3 |
3.92 |
- |
1.44 |
0.31 |
- |
- |
- |
1.05 |
- |
| Clementine |
39.9 |
4.64 |
0.08 |
- |
- |
1.25 |
0.2 |
0.5 |
- |
19.23 |
| Lemon |
20.5 |
- |
- |
- |
16.17 |
3.12 |
4.95 |
1.17 |
- |
- |
| Grapefruit |
0.93 |
7.60 |
23.0 |
0.3 |
0.41 |
- |
- |
- |
- |
- |
4. Citrus Flavonoid Extraction Techniques
Citrus flavonoids were discovered to be omnipresent in practically all portions of
Citrus fruits from various species [
45]. Extraction is a crucial stage in the analytical process, and its success has a significant impact on the quality of the final results [
46]. Flavonoids can only be isolated, detected, and characterized after using the appropriate extraction procedure. Generally, to extract bioactive compounds, several processes can be used, many of which have largely remained constant for hundreds of years. All of these strategies share the same goals: (a) extracting selected bioactive chemicals from complicated plant samples; (b) improving analytical method selectivity and avoiding the presence of interferents that could alter the analysis; and (c) improving bioassay sensitivity by increasing the concentration of targeted compounds before analysis [
46,
47,
48].
4.1. Conventional Extraction Techniques
Various traditional extraction procedures can be used to extract bioactive chemicals from plant sources. The recovery of bioactive chemicals from plant matrices, using common solvents, is referred to as conventional extraction (with or without heat treatment) [
49]. The majority of these approaches rely on the extraction power of the various solvents in use, as well as the use of heat and/or mixing. The known conventional procedures for extracting bioactive chemicals from plants are (1) maceration, (2) infusion, (3) decoction, (4) hot continuous extraction (Soxhlet extraction), (5) hydrodistillation, and (6) percolation.
4.2. Non-Conventional Extraction Techniques
Degradation of targeted compounds due to high temperatures and long extraction times in solvents is a major problem encountered in classic extraction techniques. On this basis, finding various extraction strategies to overcome this difficulty becomes a critical step to improve extraction efficiency and/or selectivity. Or, using dedicated aids/energy-intensive intrants, such as microwave-assisted extraction [
50], pressurized liquid extraction [
51], supercritical fluid extraction [
52], ultrasound-assisted extraction, cold plasma-assisted extraction [
53], high pressure assisted extraction [
54], pulsed electric field assisted extraction [
55], and enzyme-assisted extraction [
56], is well documented in the scientific literature as an efficient alternative. In general, while studying plant-derived chemicals, the method and solvents utilized for extraction must be carefully adopted [
57]. In this context, some of the non-conventional extraction methods are discussed.
4.2.1. Ultrasound Assisted Extraction (UAE)
Ultrasound-assisted extraction is a new technology that is being used to extract natural products that previously took many hours to extract using traditional methods. Initially, it was employed to preserve food, but in the last decade, it has also been utilized to extract beneficial substances (mainly polyphenols). Because of the simplicity of the method, benefits such as reduced extraction time, increased extract yield, and the use of water as a solvent, which reduces the usage of organic solvents, are documented. Therefore, to avoid unwanted reactions generated by the UAE and maximize the extraction yield, the extraction parameters (e.g., extraction duration, solvent system, and, if possible, U.S. frequency) should be tuned prior to developing the extraction process [
58]. Londoño-Londoño et al. 2010, conducted the extraction of
Citrus peel flavonoids from
C. sinensis,
C. latifolia, and
C. reticulata under optimal ultrasonic conditions of 60 kHz, 40 °C, for 1 h, using methanol as a solvent [
59].
4.2.2. Supercritical Fluid Extraction (SFE)
Supercritical extraction is a modern technique that uses gases that have exceeded their critical pressure and temperature, resulting in a fluid with qualities in between a gas and a liquid [
60]. Supercritical CO
2 extraction (using CO
2 as a solvent, mainly due to its adaptability, availability, and low cost), is a preferred approach for extracting numerous active compounds. Despite the fact that any gas can be employed as a supercritical fluid [
61] because flavonoids are polar molecules, SFE requires the presence of a cosolvent, such as ethanol or methanol [
62]. A study was carried out to extract nobiletin and tangeritin from
C. depressa var Hayata. The authors tested both methanol and ethanol as solvents. Under the conditions reported in their paper, Lee et al. [
36] found that SFE provides a higher amount of flavonoids (+7%) than UAE.