Resonance Imaging (MRI) is the most widely used non-invasive technique in the primary diagnosis of glioblastoma. Although MRI provides very powerful anatomical information, it has proven to be of limited value for diagnosing glioblastomas in some situations. The final diagnosis requires a brain biopsy that may not depict the high intratumoral heterogeneity present in this tumor type.
The gold standard tracer for most PET cancer imaging is 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG), a fluorine-18 glucose analog, being the most widely used in clinical radiopharmaceutical practice, and accounting for more than 90% of total PET scans. [18F]FDG is ineffective for diagnosing gliomas due to the high glucose metabolism in the normal brain, which results in suboptimal tumor detection and delineation, especially upon treatment. An innovative option for biomarker identification in vivo is termed “immunotargeted imaging”. By merging the high target specificity of antibodies with the high spatial resolution, sensitivity, and quantitative capabilities of positron emission tomography (PET), “Immuno-PET” allows us to conduct the non-invasive diagnosis and monitoring of patients over time using antibody-based probes as an in vivo, integrated, quantifiable, 3D, full-body “immunohistochemistry” in patients.
- diagnostic imaging
- immuno-PET
- glioblastoma
- neuroimaging
- molecular imaging
- antibody
- nanobody
- theragnostic probes
1. Introduction
2. Current Status of Glioblastoma Classification and Diagnosis
3. Neuroimaging
4. Elements of Immuno-PET: Target, Antibody and Radionuclide
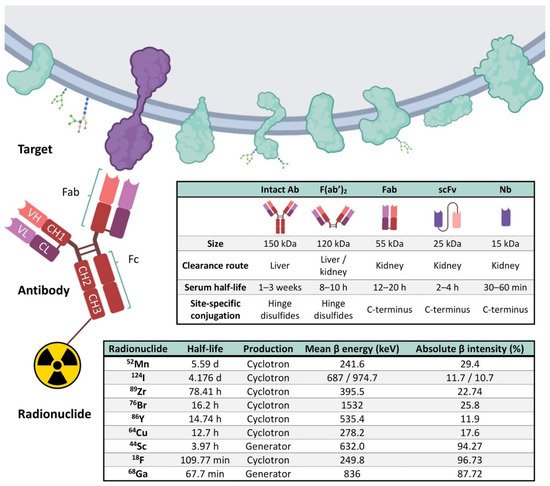
5. Current Perspectives of Immuno-PET for Glioblastoma
|
PET Imaging Probes |
Conjugation Strategy |
Targets |
Application |
Models |
References |
|---|---|---|---|---|---|
|
[18F]AlF-NOTA/NODAGA-PODS-Z-EGFR:03115 (EGFR-targeting affibody molecule) |
Cysteine-based random |
EGFR |
Many EGFR gene alterations have been identified in gliomas, especially glioblastomas. |
Subcutaneous xenograft mouse model with U-87 MG vIII cells |
[94] |
|
[124I]I-PEG4-tptddYddtpt-ch806 (tptddYddtpt is a peptide ‘‘clicked″ onto dibenzyl- clooctyne(DBCO)-derivatized ch806) |
Click chemistry |
EGFR |
ch806, an anti-EGFR mAb, can distinguish tumor cells with an amplified/overexpressed EGFR phenotype from normal cells having wild-type levels of EGFR expression. |
Subcutaneous xenograft mouse model with U-87 MG.de2-7 cells |
[95] |
|
[44Sc]Sc−CHX-A″-DTPA−Cetuximab-Fab |
Lysine-based random |
EGFR |
Radiolabeling and preclinical evaluation of 44Sc-labeled protein molecules. |
Subcutaneous xenograft mouse model with U-87 MG |
[96] |
|
[89Zr]Zr-DFO-cetuximab |
Lysine-based random |
EGFR |
89Zr-cetuximab was used to assess transient BBB disruption in vivo permeability induced by the combination of injected microbubbles with low intensity focused ultrasound. |
Orthotopic murine glioma with GL261 cells |
[97] |
|
[64Cu]Cu-NOTA-Bs-F(ab)2 (bispecific immunoconjugate by linking two antibody Fab……fragments, an anti-EGFR and an anti-CD105) |
Lysine-based random |
EGFR and CD105 |
EGFR has been extensively studied as a target for anticancer therapy, and its activation stimulates tumor proliferation and angiogenesis. Similarly, CD105 (also called endoglin) is abundantly expressed on activated endothelial cells, and such over-expression is an adverse prognostic factor in many malignant tumor types. |
Subcutaneous xenograft mouse model with U-87 MG |
[98] |
|
[64Cu]Cu-NOTA-EphA2-4B3 (human anti-EphA2 mAb) |
Lysine-based random |
EphA2 |
EphA2 receptor tyrosine kinase is overexpressed in several tumors, including glioblastoma. |
Orthotopic brain glioblastoma murine models (two patient-derived cell lines and U-87 MG cells) |
[99] |
|
[89Zr]Zr-DFO-mCD47 |
Lysine-based random |
CD47 |
CD47 is a membrane protein overexpressed on the surface of most cancer cells. It is involved in the increase in intracellular [Ca2+] that occurs upon cell adhesion to the extracellular matrix and is also a receptor for the C-terminal cell-binding domain of thrombospondin. |
Orthotopic murine glioma with GL261 cells |
[100] |
|
[64Cu]Cu-NOTA-AC133 (anti-AC133 mAb) |
Lysine-based random |
AC133 |
AC133 is an N-glycosylation-dependent epitope of the second extracellular loop of CD133/prominin-1, a cholesterol-binding protein of unknown function that locates to plasma membrane protrusions. AC133+ tumor stem cells have been described for glioblastoma multiforme. |
Orthotopic and subcutaneous xenograft mouse models with NCH421k and U-251 MG cells |
[101] |
|
[89Zr]Zr-DFO-bevacizumab (humanized anti-VEGF) |
Lysine-based random |
VEGF |
89Zr-labeled bevacizumab was used to assess BBB opening with mannitol. |
C3HeB/FeJ mice without tumors |
[102] |
|
[68Ga]Ga-DOTA-bevacizumab (humanized anti-VEGF) |
Lysine-based random |
VEGF |
68Ga-labeled bevacizumab was used to assess BBB opening with focused ultrasound exposure in the presence of microbubbles. |
Orthotopic murine glioma with U-87 MG cells |
[103] |
|
[89Zr]Zr-DFO-YY146 (anti-CD146 mAb) |
Lysine-based random |
CD146 |
CD146 plays an important role in several processes involved in tumor angiogenesis, progression, and metastasis. Its expression has been correlated with aggressiveness in high-grade gliomas. |
Subcutaneous xenograft mouse model with U-87 MG and U251 cells |
[104] |
|
[64Cu]Cu-NOTA-YY146 (anti-CD146 mAb) |
Lysine-based random |
CD146 |
CD146 plays an important role in several processes involved in tumor angiogenesis, progression, and metastasis. Its expression has been correlated with aggressiveness in high-grade gliomas. |
Orthotopic and subcutaneous xenograft mouse models with U-87 MG and U-251 MG cells |
[105] |
|
[64Cu]Cu-NOTA-61B (human anti-Dll4 mAb) |
Lysine-based random |
DII4 |
DII4 plays a key role to promote the tumor growth of numerous cancer types. |
Subcutaneous xenograft mouse model with U-87 MG |
[106] |
|
[89Zr]Zr-DFO-LEM2/15 (anti-MM1-MMP mAb) |
Lysine-based random |
MT1-MMP/ MMP14 |
MMP14 is a metalloprotease frequently overexpressed in many tumors, and it is associated with tumor growth, invasion, metastasis, and poor prognosis. |
Xenograft mice bearing human U251 cells and two orthotopic brain glioblastoma murine models (patient-derived TS-543 neurospheres and U-251 MG cells) |
[107] |
|
[89Zr]Zr-DFO-fresolimumab (human IgG4 mAb, 1D11) |
Lysine-based random |
TGFβ |
TGFβ mediates extracellular matrix (ECM) remodeling, angiogenesis, and immunosuppression, and regulates tumor cell motility and invasion. |
Orthotopic murine glioma with GL261 and SB28 cells |
[108] |
|
[89Zr]Zr-DFO-fresolimumab (human IgG4 mAb, 1D11) |
Lysine-based random |
TGFβ |
TGFβ mediates ECM remodeling, angiogenesis, and immunosuppression, and regulates tumor cell motility and invasion. |
Patients with recurrent high-grade glioma |
[109] |
|
[89Zr]Zr-DFO-F19 (anti-FAP monoclonal antibody) |
Lysine-based random |
FAP |
FAP, a 170 kDa type II transmembrane serine protease, is expressed on glioma cells and within the glioma tumor microenvironment. |
Subcutaneous xenograft mouse model with U-87 MG cells |
[110] |
|
[89Zr]Zr-DFO-PD-1 |
Lysine-based random |
PD-1 |
89Zr labeled αPD-1 antibody was used to assess focal BBB permeability induced by high-intensity, focused ultrasound. |
Orthotopic murine glioma with G48a cells |
[111] |
|
[68Ga]Ga-NOTA-Nb109 (anti-PD-L1 nanobody) |
Lysine-based random |
PD-L1 |
Evaluate the specific affinity of 68Ga-NOTA-Nb109 to several cancer cell lines that expressed endogenous PD-L1. |
Subcutaneous xenograft mouse model with U-87 MG cells |
[112] |
|
[89Zr]Zr-DFO-169 cDb (anti-CD8 cys-diabody) |
Lysine-based random |
CD8 |
Proof-of-concept to detect CD8+ T cell immune response to oncolytic herpes simplex virus (oHSV) M002 immunotherapy in a syngeneic glioblastoma model. |
Orthotopic syngeneic murine glioma with GSC005 cells |
[113] |
|
[89Zr]Zr-DFO-CD11b |
Lysine-based random |
CD11b |
The most abundant population of immune cells in glioblastoma is the CD11b+ tumor-associated myeloid cells. |
Mice bearing established orthotopic syngeneic GL261 gliomas |
[114] |
|
[89Zr/177Lu]Zr/Lu-Lumi804-CD11b |
Lysine-based random |
CD11b |
Theragnostic approach for monitoring and reducing tumor-associated myeloid cells in gliomas to improve immunotherapy responses. |
Mice bearing established orthotopic syngeneic GL261 gliomas |
[115] |
|
[89Zr]Zr-DFO-OX40 |
Lysine-based random |
CD134 |
CD134 (or OX40) is an activated T-cell surface marker, known to be a costimulatory transmembrane molecule of TNF superfamily, primarily expressed on activated effector T cells and regulatory T cells. |
Mice bearing established orthotopic GL261 gliomas |
[116] |
6. Novel Nanobody-Based Immuno-PET Imaging Methods for Glioblastoma
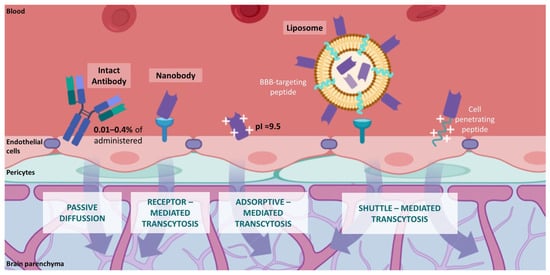
This entry is adapted from the peer-reviewed paper 10.3390/cancers14010074
