Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
The silica hydride surface is composed of silicon-hydrogen groups, which is much more stable, less reactive and delivers different chromatographic and chemical characteristics.
- HPLC
- aqueous normal-phase
- dual retention modes
- surface water layer
1. Salient Features of Silica Hydride Stationary Phases
The bonding technology used to make these materials, hydrosilation, is completely different than that used for columns based on ordinary silica, organosilanization. The latter utilizes an organoilane reagent that reacts with the silanols on the surface to bond an organic moiety. Hydrosilation involves reacting an olefin or alkyne compound with the Si-H group to attach the organic species to the surface. The production of silica hydride results in approximately the same number of Si-H groups on the surface as ordinary silica has silanols. However, at the end of the bonding reactions, the remaining groups on the stationary phase are polar silanols for ordinary silica but nonpolar Si-H for the silica hydride phases. For silica hydride phases, the organic moiety is attached via a direct Si-C, while for ordinary silica, the linkage is Si-O-Si-C. The direct silicon-carbon bond provides much higher stability than the siloxane linkage obtained with organisilanization. In addition, the hydrosilation reactions have proven to be robust and reproducible (Scheme 1). For the same analysis on different lots of the same column material, variations in retention times or capacity factors (k) are no more than a few tenths of a percent RSD.
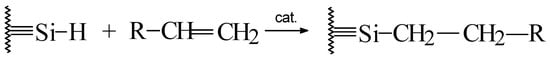
Scheme 1. Hydrosilation reaction.
There are numerous features that distinguish silica hydride from the ordinary silica used to fabricate the vast majority of commercially available HPLC stationary phases. The most obvious of these differences are the surface properties of these two materials. The surface of ordinary silica is populated by polar silanol groups (Si-OH) while the surface of silica hydride is composed of nonpolar silicon hydride moieties (Si-H). This profound difference has significant ramifications when comparing the two materials with respect to physical properties and chromatographic behavior. Because of the polar nature of silanols, many analytes can be adsorbed on the surface of stationary phases fabricated on ordinary silica. Even extensive endcapping leaves a significant number of silanols on the surface. However, silica hydride materials are composed of almost entirely Si-H groups that precludes the adsorption of most analytes on the surface. Another consequence of this difference in surfaces is that ordinary silica strongly adsorbs water leading to an aqueous layer of at least 4–10 monolayers in contrast to silica hydride having less than a monolayer of water under most chromatographic conditions [1][2]. This is especially significant when silica-based materials are used in the hydrophilic interaction liquid chromatography (HILIC) mode. Most HILIC analyses depend on a partitioning of analytes from the mobile phase to this adsorbed water layer as a means of chromatographic retention. Since such a layer does not exist on silica hydride materials, these phases utilize other mechanisms for polar compound retention (see discussion below). Another consequence of the lack of a water layer is the rapid equilibration between runs in gradient analysis or rapid equilibration when mobile phase conditions are changed for different analyses or method development. Another ramification of the absence of a significant water layer on the silica hydride surface is evident in organic normal phase chromatography. Since ordinary silica readily absorbs water, it must be carefully removed from the mobile phase; otherwise, the results can vary significantly from run-to-run on both an intraday and interday basis. However, the low affinity for water on a silica hydride surface eliminates the need to scrumptiously dry mobile phase solvents for organic normal phase chromatography.
The retention for most nonpolar compounds on silica hydride columns is similar to that for ordinary silica-based stationary phases, i.e., hydrophobic interactions between the analyte and a bonded organic moiety such as C18 or C8. Thus, typical reversed-phase HPLC analyses can be done on these types of silica hydride phases. However, the mechanism of retention for polar compounds on silica hydride stationary phases was an issue for many years, since the surface is hydrophobic, yet strong retention of hydrophilic analytes was observed for a broad range of compounds in the aqueous normal phase mode. It was determined [3] that in mobile phases with a high content of an organic solvent such as acetonitrile, auto-dissociation of water occurs on silica hydride with hydroxyl ions prevalent on the surface of the material, giving it a negative overall charge. This phenomenon is similar to what happens to oil droplets in mixed solutions of organic solvent and water. Thus, retention of compounds having a positive charge or those having a positively polar component such as an amine group occurs by charge attraction and negatively charged or polarized molecules by displacement.
The ability to retain polar and nonpolar compounds applies to all stationary phases created on a silica hydride surface. A plot of retention as a function of the percentage of organic component in the mobile phase results in a U-shaped curve (Figure 1). On the left-hand side of the graph, at low organic or high water content, reversed-phase retention is observed. On the right-hand side, at high organic or low water content, normal-phase retention is obtained. At intermediate mobile phase compositions, it is possible to have both reversed-phase and normal-phase retention operating simultaneously. For compounds with significant polar and nonpolar components in its structure, retention can be achieved in either mode, thus giving the analyst more options in developing a suitable method. This is a hypothetical graph, and the exact shape depends on both the analyte and the stationary phase. Other phases, such as certain fluorinated bonded compounds, also display this behavior to a limited extent in comparison to the broad range of retention properties exhibited by silica hydride-based materials. It has also been demonstrated that silica hydride can be prepared under supercritical fluid conditions but only in very small quantities [4].
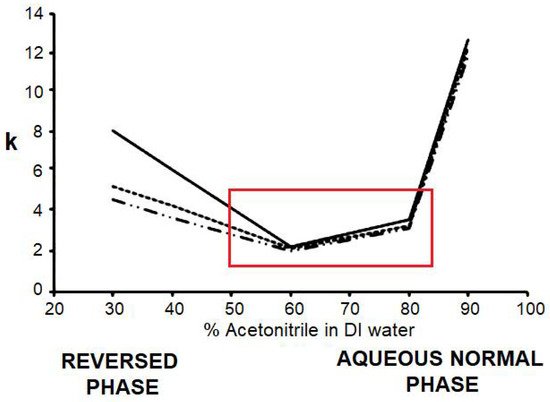
Figure 1. Retention map (k vs. % organic in mobile phase) for analytes on a silica hydride-based column.
2. Significant Applications by Column Type
While all silica hydride columns can operate in both the reversed-phase and normal-phase modes, the bonded moiety usually determines what retention properties will predominate. Thus, a nonpolar bonded moiety such as C18 will generally be used for reversed-phase applications, but it still has normal-phase properties in contrast to most other stationary phase with this type of modification. More polar bonded groups, such as diol or amide, would generally be used in normal-phase applications, but it still retains some reversed-phase properties. The following sections contain applications for each type of commercially available silica hydride column, which at this time happens to be produced by only one company. Columns types that are not available include chiral, size exclusion, anion-exchange, gel permeation, sub two-micron particle size and core shell.
2.1. Diamond Hydride
This is the most widely used of the silica hydride-based columns. It was the first stationary phase to display capabilities for analyzing a broad range of polar compounds. Even today, it has features that either are not available or less versatile than most HILIC phases. The ability of equilibrate rapidly in normal-phase applications under a variety of mobile phase conditions (usually two to three column volumes) is in most cases vastly superior to HILIC columns. Another aspect of the Diamond Hydride column is its use of low buffer concentrations in the mobile phase. Typical additive concentrations are in the 5 to 15 mM range, while many HILIC applications can be significantly higher (30 to 100 mM is not unusual). This can be particularly important when using mass spectrometry for detection. High additive concentrations can leave deposits on the ion source, thus lowering sensitivity and requiring frequently cleaning. While acetonitrile is the most common organic solvent used in ANP analyses on the Diamond Hydride column, acetone [5] and methanol [6] have been shown to be applicable as well. Another feature of this stationary phase is its ability to retain certain strongly polar compounds in the normal-phase mode at very low concentrations of organic in the mobile. This often occurs at 20 to 30% organic content and has been referred to as “super ANP”.
The analysis of polar compounds is an important component that occurs in a variety of different application areas, such as pharmaceuticals, metabolites, food, forensics, environment, and biotechnology. Many examples describing a variety of analyses of hydrophilic compounds can be found in the literature as well as on the Microsolv Technology website [7]. Some examples of the approximately 200 listed application notes through the website are the analysis of hydrophobic and hydrophilic peptides in a single run [8], benzodiazepines in urine [9], acrylamide [10], the pharmaceutical Xanax [11], anatoxin, which is a neurotoxin implicated in many poisoning incidents [12], and the common household product ingredient cetylpyridinium chloride [13]. Some examples of hydrophilic compounds analyzed on the Diamond Hydride in the literature are common metabolites [5], cathinones [14], collagen and elastin crosslinks [6], bactericidal targets [15], sugars [16], lipids [17], thiopurines [18], dietary supplements [19], juices and cereals [20], peptides [21], nucleotides [22], drug levels in human serum [23], and disease pathways [24][25][26]. In another report, the Diamond Hydride was combined with an RP column for a comprehensive survey of both polar and nonpolar metabolites [27]. One study compared the Diamond Hydride and three other Type-C stationary phases with respect to the relative retention of small molecules [28]. This range of applications displays the versatility of the Diamond Hydride column for polar compound analysis that goes beyond the capability of typical HILIC phases.
2.2. Phenyl
The phenyl phase having a hydrophobic moiety bonded to the silica hydride surface is generally used for reversed-phase applications, particularly those where the analytes contain aromatic or other types of unsaturated sites. Retention is enhanced for these types of analytes though π-π interactions with the bonded moiety.
An example of an application for the phenyl column in the literature is the analysis of 16 common drugs of abuse by LCMS in under 8 min [29]. Under a different set of experimental conditions, the THC-delta-9-COOH can also be analyzed on the phenyl column. Additional articles citing the use of phenyl hydride columns involve the analysis of rice [30], mycotoxins in grains [31], and jaboticaba fruit [32]. However, there are more than 30 examples of analyses using the phenyl hydride column on the Microsolv website [33]. Included among the applications presented are the analysis of 10 phenolic acids in rice, methylenedioxymethamphetamine (MDMA) in plasma and the pharmaceutical compounds coricidin, fluoxetine, and ketorolac. An interesting example is shown in Figure 2 for the analysis of common components found in cough syrup [33]. This is a gradient analysis that is an overlay of five consecutive runs with an equilibration time of 3 min between runs. The run-to-run reproducibility of Cogent columns is one of their essential features, as well as rapid column equilibration between gradient runs.
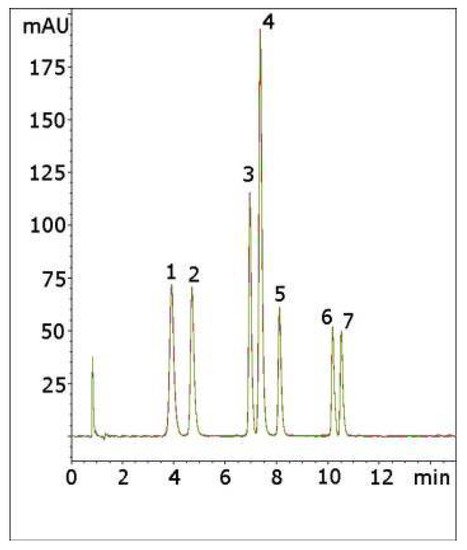
Figure 2. Gradient separation of common components found in cough syrup. Column, Cogent Phenyl Hydride 4.6 × 75 mm. Mobile phase, A DI water/0.1% TFA and B acetonitrile/0.1% TFA. Gradient: 0–2 min 5% B, 2–11 min to 50% B, 11–12 min to 5% B. Analytes: 1 = acetaminophen; 2 = pseudoephedrine; 3 = guafenesin; 4 = benzoic acid; 5 = methylparaben; 6 = dextromethorphan; and 7 = propyl paraben. Overlay of two column lots.
2.3. Amide
A more recently developed silica hydride column is the amide stationary phase. The amide phase focuses primarily on hydrophilic molecules and is especially applicable to the analysis of sugars and various carbohydrates. Both a fundamental description of this phase and some examples of applications can be found in a published article [34].
Additional information on applications of the silica hydride-based amide column can be found online. A range of analyses are presented, relating to compounds that are not carbohydrates. An interesting example is the determination of the antidepressant fluoxetine (Prozac) in a capsule [35]. The compound is polar with a secondary amine, an ether linkage, and a trifluoromethyl group. The lot-to-lot reproducibility of this phase is also demonstrated in the application with three synthetic batches showing virtually identical retention times for the analyte. Nitrogen-containing compounds can often be difficult to analyze in reversed-phase due to adsorption on residual silanols and is often challenging in the HILIC mode. The separation of pyrilamine and 4-amino-3-chloropyridine with good peak shape demonstrates the ability of the silica hydride-based amide column for these types of compounds [35]. Another application involves the analysis of the pharmaceutical compound tizanidine in tablet form used to treat muscle spasms and cramps [35]. A particularly interesting application is shown Figure 3 for the separation of glucose and fructose in cola. These structurally similar compounds are a challenging separation, but can be done in the ANP mode under isocratic conditions using the amide silica hydride column [35].
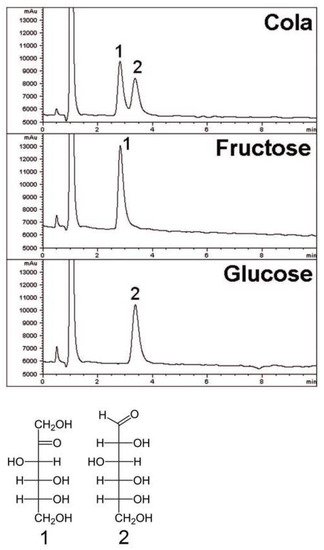
Figure 3. Separation of fructose and glucose on the silica hydride-based amide column. Column 4.6 × 50 mm. Mobile phase: 5% DI Water/95% acetonitrile.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26247505
References
- Soukup, J.; Janas, P.; Jandera, P. Gradient elution in aqueous normal-phase liquid chromatography on hydrosilated silica-based stationary phases. J. Chromatogr. A 2013, 1216, 111–116.
- Bartó, E.; Felinger, A.; Jandera, P. Investigation of the temperature dependence of water adsorption on silica-based stationary phases in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2017, 1489, 143–148.
- Kulsing, C.; Nolvachi, Y.; Marriott, P.; Boysen, R.; Matyska, M.; Pesek, J.; Hearn, M. Insights into the origin of the separation selectivity with silica hydride adsorbents. J. Phys. Chem. Part B 2015, 119, 3063–3069.
- Scully, N.M.; Ashu-Arrah, B.A.; Nagle, A.P.; Omamogho, J.O.; O’Sullivan, G.P.; Friebolin, V.; Dietrich, B.; Albert, K.; Glennon, J.D. Silica hydride intermediate for octadecylsilica and phenyl bonded phase preparation via heterogeneous hydrosilation in supercritical carbon dioxide. J. Chromatogr. A 2011, 1218, 1974–1982.
- Pesek, J.J.; Matyska, M.T.; Fischer, S.M.; Sana, T.R. Analysis of hydrophilic metabolites by high-performance liquid chromatography—Mass spectrometry using a silica hydride-based stationary phase. J. Chromatogr. A 2008, 1204, 48–55.
- Naffa, R.; Holmes, G.; Ahn, M.; Harding, D.; Norris, G. Liquid chromatography-electrospray ionization mass spectrometry for the simultaneous quantitation of collagen and elastin crosslinks. J. Chromatogr. A 2016, 1478, 60–67.
- Available online: https://kb.mtc-usa.com/category/46/63/91/cogent-type-c-silica-columns/90/ (accessed on 8 November 2021).
- Available online: https://kb.mtc-usa.com/article/aa-03183/63/ (accessed on 8 November 2021).
- Available online: https://kb.mtc-usa.com/article/aa-02318/46/ (accessed on 8 November 2021).
- Available online: https://kb.mtc-usa.com/article/aa-00950/46/ (accessed on 8 November 2021).
- Available online: https://kb.mtc-usa.com/article/aa-02342/46/ (accessed on 8 November 2021).
- Available online: https://kb.mtc-usa.com/article/aa-00807/46/ (accessed on 8 November 2021).
- Available online: https://kb.mtc-usa.com/article/aa-01023/46/ (accessed on 8 November 2021).
- Ploumen, C.; Marginean, J.; Lurie, I.S. The utility of silica hydride-based stationary phases for dual-mode ultra high performance liquid chromatography separation of synthetic cathinone positional isomers. J. Sep. Sci. 2020, 43, 3449–3457.
- Evans, J.C.; Trujillo, C.; Wang, Z.; Eoh, H.; Ehrt, S.; Schnappinger, D.; Boshoff, H.I.M.; Rhee, K.Y.; Barry III, C.E.; Mizrahi, V. Validation of CoaBC as a bactericidal target in the coenzyme A pathway of Mycobacterium tuberculosis. ACS Infect. Dis. 2016, 2, 958–968.
- Young, J.E.; Pesek, J.J.; Matyska, M.T. Robust HPLC-refractive index analysis of simple sugars in beverages using silica hydride columns. Curr. Nutr. Food Sci. 2016, 12, 125–131.
- Cífková, E.; Hájek, R.; Lísa, M.; Holčapek, M. Hydrophilic interaction liquid chromatography–mass spectrometry of (lyso)phosphatidic acids, (yso)phosphatidylserines and other lipid classes. J. Chromatogr. A 2016, 1439, 65–73.
- Pesek, J.J.; Matyska, M.T.; Young, J.E. Analysis of thiopurines using aqueous normal phase chromatography. J. Pharm. Biomed. Anal. 2014, 95, 102–106.
- Le, R.; Young, J.E.; Pesek, J.J.; Matyska, M.T. Separation of 1,3-dimethylamylamine and other polar compounds in dietary supplement formulation using aqueous normal phase chromatography with mass spectrometry. J. Sep. Sci. 2013, 36, 2578–2583.
- Young, J.E.; Matyska, M.T.; Pesek, J.J. Liquid chromatography/mass spectrometry compatible approaches for the quantitation of folic acid in fortified juices and cereals using aqueous normal phase conditions. J. Chromatog. A 2011, 1218, 2121–2126.
- Boysen, R.I.; Yang, Y.; Chowdhury, J.; Matyska, M.T.; Pesek, J.J.; Hearn, M.T.W. Simultaneous separation of hydrophobic and hydrophilic peptides with a silica hydride stationary phase using aqueous normal phase conditions. J. Chromatogr. A 2011, 1218, 8021–8026.
- Pesek, J.J.; Matyska, M.T.; Duley, J.; Zamzami, M.; Fischer, S.M. Aqueous normal phase (ANP) retention of nucleotides on silica hydride-based columns. Method development strategies for analytes relevant in clinical analysis. J. Sep. Sci. 2010, 33, 930–938.
- Ge, J.; Liu, F.; Holmes, E.H.; Ostrander, G.K.; Li, Q.X. Aqueous normal phase liquid chromatography coupled with tandem time-of-flight quadrupole mass spectrometry for determination of zanamivir in human serum. J. Chromatogr. B 2012, 906, 58–62.
- Villarino, G.; Guerquin-Kern, J.-L.; Sório de Carvalho, L.P.; Poquet, Y.; Neyrolles, O. Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat. Chem. Biol. 2013, 9, 674–676.
- Sana, T.R.; Gordon, D.B.; Fischer, S.M.; Tichy, S.E.; Norton Kitagawa, N.; Lai, C.; Gosnell, W.L.; Chang, S.P. Global Mass Spectrometry Based Metabolomics Profiling of Erythrocytes Infected with Plasmodium falciparum. PLoS ONE 2013, 8, e60840.
- Marcobal, A.; Kashyap, P.C.; Nelson, T.A.; Aronov, P.A.; Donia, M.S.; Spormann, A.; Fischbach, M.A.; Sonnenburg, J.L. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013, 7, 1933–1943.
- Chalcraft, K.R.; McCarry, B.E. Tandem LC columns for the simultaneous retention of polar and nonpolar molecules in comprehensive metabolomics analysis. J. Sep. Sci. 2013, 36, 3478–3485.
- Appulage, D.K.; Schug, K.A. Silica hydride based phases for small molecule separations usingautomated liquid chromatography–mass spectrometry method development. J. Chromatogr. A 2017, 1507, 115–123.
- Pesek, J.J.; Matyska, M.T.; Kim, A.M. Evaluation of silica hydride-based stationary phases for the analysis of drugs of abuse. J. Sep. Sci. 2013, 36, 2760–2766.
- Villanova, F.A.; Vanier, N.L.; Chaves, F.C.; Pesek, J.; Matyska-Pesek, M.; Elias, M.C.; de Oliveir, M. Improvement of the quality of parboiled rice by using anti-browning agents during parboiling process. Food Chem. 2017, 235, 51–57.
- Pesek, J.; Matyska, M.; Hoffman, J.F.; Madruga, N.; Crizel, R.L.; Elias, M.C.; Vanier, N.L.; Chaves, F.C. Analysis of mycotoxins in grains using silica hydride-based stationary phases. J. Sep. Sci. 2017, 40, 1953–1959.
- Watanabe, S.; Matyska-Pesek, M.T.; Berrious, J.D.J.; Takeoka, G.R.; Pesek, J.J. HPLC/ESI-TOF-MS identification and quantification of phenolic compounds in fermented/nonfermented jaboticaba fruit (myrciaria jaboticaba (vell.) O. berg). Int. J. Food Sci. Nutr. 2018, 3, 105–109.
- Available online: https://kb.mtc-usa.com/category/46/63/91/cogent-type-c-silica-columns/93/ (accessed on 8 November 2021).
- Pesek, J.J.; Matyska, M.T.; Tardiff, E.; Hiltz, T. Chromatographic characterization of a silica hydride-based amide stationary phase. J. Sep. Sci. 2021, 44, 2728–2734.
- Available online: https://kb.mtc-usa.com/category/46/63/91/cogent-type-c-silica-columns/167/ (accessed on 8 November 2021).
This entry is offline, you can click here to edit this entry!
