Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
|
Neurosciences
The peripheral nervous system (PNS) exhibits a limited capacity for functional and morphological repair and regeneration. Peripheral nerve recovery is a multistep process with a complex molecular and cellular regulatory circuitry. Severe injury of peripheral nerves often results in a loss of motor, sensory, and autonomic functions of innervated organs and tissues, therefore calling for novel treatment strategies to ensure effective regeneration.
- peripheral nerve regeneration
- axon growth
- Schwann cells
- exosomes
- microRNA
1. Cell Adhesion Molecules and Extracellular Matrix Proteins in Peripheral Nerve Regeneration
Peripheral nerve repairment relies on the ability of regenerating axons to navigate through a structurally altered ECM, myelin, and cell debris, infiltrating inflammatory cells. During early Wallerian degeneration, not only do the axons degenerate and Schwann cells adopt transdifferentiation, but ECM undergoes a substantial degradation thus removing the inhibitory influence of chondroitin-sulfate proteoglycans and myelin-associated glycoprotein, predominantly expressed in the matrix of intact axons, and facilitating the new axon sprouting [1]. The spatio-temporally relevant degradation of ECM provided by the activated proteases is the limiting factor of regenerating axonal growth. Pro-inflammatory cytokines such as HIF-1 and TNF-α induce secretion of plasminogen activators (PAs) and matrix metalloproteinases (MMPs) by neurons, Schwann cells, and macrophages involved in matrix remodeling [2][3].
Following ECM degradation, Schwann cells and fibroblasts rapidly up-regulate the production of new ECM and basement membrane proteins at the site of injury, with laminin, fibronectin, and collagen being predominant types promoting nerve regeneration. This change in the ECM composition promotes the axon growth-cone formation and axon elongation with new integrin-mediated adhesive contacts playing an important role in regenerating peripheral axons. In particular, cytoskeleton proteins undergo qualitative and quantitative changes characterized by an increased expression of actin, tubulin, and peripherin, and a decrease in neurofilaments regulating the axon caliber [4]. The mobile path-finder parts of the growth-cone filopodia and lamellipodia are constantly searching for a permissive substrate and, once encountering the growth-cone receptors, integrins bind to ECM and form adhesions. Integrins are heterodimeric receptors consisting of α and β subunits with a unique affinity of each integrin receptor for different ECM proteins. Specifically, type I, III, and V collagens interact with α1β1 and α2β1 integrins, while α4β1, α5β1, and α8β1 integrins mainly bind fibronectin [5].
2. Guidance Molecules in Nerve Regeneration
Studies on peripheral nerve regeneration in adults have revealed a key role of signaling cascades downstream of the guidance receptors, such as EphrinB2, Slit3, and Netrin1 [6]. After the peripheral nerve injury, Netrin-1 expression is significantly elevated in Schwann cells migrating from the distal stump, in the cell somas of sensory neurons, and in the axons of both motor and sensory neurons. Netrin-1 interacts with Neogenin on Schwann cells and CD146 receptor on endothelial cells, boosting their proliferation and migration. Netrin-1 can also contribute to axon elongation through interaction with receptors DCC (Deleted in Colorectal Cancer) along with other molecules, such as Neogenin, DSCAM and CD146 on the growth cone of regenerating axons. Interestingly, Netrin-1 can act as a bifunctional axon guidance cue: Netrin-1-DCC binding results in axon attraction, while Netrin-1-Unc5A–D (Uncoordinated receptor 5A–D) interaction leads to axon repulsion and slows down the rate of axon growth during regeneration, adding further complexity to the regulatory circuitry [7].
Schwann cells migrating from the injured nerve stumps and expressing EphB2 come into a direct contact with fibroblasts expressing EphrinB2. This interaction results in Ephrin-B/EphB2-mediated cell sorting of these two types, which in turn coordinates migration of Schwann cells as multicellular Büngner bands and guides the axonal growth across the injury site [8].
3. Neurotrophins and Cytokines in Peripheral Nerve Regeneration
Neurotrophic factors are a heterogeneous group of signaling molecules that play a pivotal role in PNS development, maintenance, and plasticity. The group comprises three multigene families—the neurotrophins (NGF, BDNF, NT-3, and NT-4/5), neuropoetic cytokines (IL-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M (OSM), CNTF), and the glial cell line-derived neurotrophic factor ligands (GDNF, neurturin, artemin, and persephin) [9].
Previous studies have shown that growth factors and cytokines are intricately involved in the axon regeneration, formation of Büngner bands, and neuronal survival [10]. When sensory and motor neurons are injured, several growth neurotrophic factors and cytokines are up-regulated or activated in neurons and the surrounding tissues. The change in the expression pattern largely relies on the specific type of the damaged neurons. In particular, it has been revealed that NGF and BDNF expression is up-regulated in sensory neurons [11] and NT-3 in motoneurons [12], while GDNF exert a pronounced trophic effect on sensory neurons [13]. Along with that, the expression of TrkA and TrkB, which are high-affinity receptors for neurotrophins NGF, BDNF, and NT-4, correspondingly, is elevated on the growth cone of the regenerating axons [14]. BDNF and GDNF expression has been reported to be also augmented in Schwann cells. Interestingly, while Schwann cells do not express high-affinity receptors, they do express a low-affinity receptor p75—a common receptor for all neurotrophins (NGF, BDNF, NT3, NT4) [10].
4. The Role of Exosomes in Regeneration of Peripheral Nerves
Recent advances in cell and molecular biology highlighted a novel mechanism that ensures intercellular communication via exosomes known to be secreted by all cell types [15][16]. As of today, a large body of evidence indicates that exosomes play a pivotal role in transferring information via their highly selective cargo. Exosomes comprising regulatory and signaling molecules can be released from the source cells of one type and fuse with the target cells of another type, therefore assisting in the integrated intercellular transfer of information [17]. The content of cargo molecules in exosomes is critically dependent upon their cell origin and physiological or pathological conditions at the moment of exosome formation. Cargo molecules are packed during exosome biogenesis and comprise proteins such as tetraspanins (CD9, CD63, CD81, and CD82), Alix, tumor susceptibility gene 101 (TSG101), Rab GTPases, heat shock proteins (Hsp70, Hsp90), cell adhesion molecules, cytoskeleton proteins, cytokines, regulatory proteins, transcription factors; lipids and genetic cargoes including DNA, mRNA, microRNA, ribosomal RNA (rRNA), circular RNA, long noncoding RNA (lnRNA) (Figure 1) [18][19].
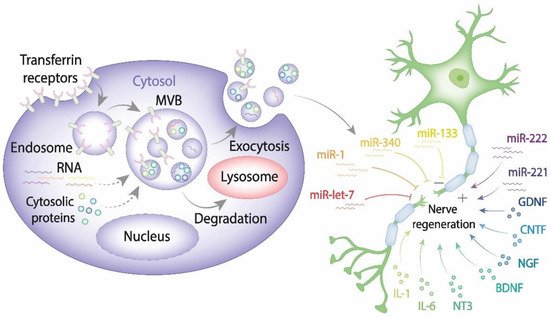
Figure 1. The exosome contribution to nerve regeneration. Exosomes are generated from late endosomes, which are formed by inward budding of the limited multivesicular body (MVB) membrane. The MVBs can either fuse with lysosomes for degradation or with the plasma membrane, therefore releasing exosomes into the extracellular space. Released exosomes taken up by damaged neurons and Schwann cells can either enhance or impair PNS.
5. The Role of miRNAs in the Regeneration of Peripheral Nerves
MicroRNAs (miRNAs) are short (~22 bp) single-stranded noncoding RNAs which function by a partial binding (partial complementarity) to mRNA. MiRNAs are currently considered to be “master regulators” of gene expression that orchestrate protein expression at the posttranscriptional level by binding to the 3′UTR of target messenger RNA, either hindering mRNA translation or inducing mRNA degradation. For almost 30 years since miRNAs were discovered, they have received widespread attention due to their potential role in a wide variety of physiological and pathological processes, including neurogenesis, neuronal maturation, embryogenesis, and regeneration of the nervous system among other complicated biological processes. MiRNAs can affect the phenotype of both the recipient and donor cells [20][21].
6. The Role of Exosomes Derived from Mesenchymal Stem Cells (MSCs) in Peripheral Nerve Regeneration
MSCs are self-renewing multipotent progenitors that can be found in various tissues and organs: bone marrow, adipose tissue, dental pulp, umbilical cord blood, etc. An extensive body of literature indicates that MSCs of different origin have the capacity to promote tissue repair and neuroprotection in vivo and in vitro. Specifically, MSCs promote axonal growth, maintain neuronal survival, and significantly improve functional nerve recovery after injury [22][23][24].
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413380
References
- Chernousov, M.A.; Yu, W.M.; Chen, Z.L.; Carey, D.J.; Strickland, S. Regulation of Schwann cell function by the extracellular matrix. Glia 2008, 56, 1498–1507.
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology 2005, 7, 134–153.
- Tang, B.L. Inhibitors of neuronal regeneration: Mediators and signaling mechanisms. Neurochem. Int. 2003, 42, 189–203.
- Zheng, J.Q.; Kelly, T.K.; Chang, B.; Ryazantsev, S.; Rajasekaran, A.K.; Martin, K.C.; Twiss, J.L. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001, 21, 9291–9303.
- Letourneau, P.C.; Shattuck, T.A. Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development 1989, 105, 505–519.
- Dun, X.-P.; Parkinson, D. Classic axon guidance molecules control correct nerve bridge tissue formation and precise axon regeneration. Neural Regen. Res. 2020, 15, 6–9.
- Dun, X.P.; Parkinson, D.B. Role of Netrin-1 Signaling in Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 491.
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155.
- Ogier, M.; Kron, M.; Katz, D.M. Neurotrophic factors in development and regulation of respiratory control. Compr. Physiol. 2013, 3, 1125–1134.
- Gordon, T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J. Commun. Disord. 2010, 43, 265–273.
- Terada, Y.; Morita-Takemura, S.; Isonishi, A.; Tanaka, T.; Okuda, H.; Tatsumi, K.; Shinjo, T.; Kawaguchi, M.; Wanaka, A. NGF and BDNF expression in mouse DRG after spared nerve injury. Neurosci. Lett. 2018, 686, 67–73.
- Sterne, G.D.; Coulton, G.R.; Brown, R.A.; Green, C.J.; Terenghi, G. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J. Cell Biol. 1997, 139, 709–715.
- Erickson, J.T.; Brosenitsch, T.A.; Katz, D.M. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J. Neurosci. 2001, 21, 581–589.
- Raivich, G.; Kreutzberg, G.W. Pathophysiology of glial growth factor receptors. Glia 1994, 11, 129–146.
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321.
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.; Fernandes, J.; Pochampally, R.; et al. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cell Int. 2016, 2016.
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil. Neural Repair 2018, 32, 765–776.
- Wahlgren, J.; Statello, L.; Skogberg, G.; Telemo, E.; Valadi, H. Delivery of Small Interfering RNAs to Cells via Exosomes. In SiRNA Delivery Methods: Methods and Protocols; Shum, K., Rossi, J., Eds.; Springer: New York, NY, USA, 2016; pp. 105–125.
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzi, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761.
- Wu, D.; Murashov, A. Molecular mechanisms of peripheral nerve regeneration: Emerging roles of microRNAs. Front. Physiol. 2013, 4.
- Semina, E.V.; Rysenkova, K.D.; Troyanovskiy, K.E.; Shmakova, A.A.; Rubina, K.A. MicroRNAs in Cancer: From Gene Expression Regulation to the Metastatic Niche Reprogramming. Biochemistry 2021, 86, 785–799.
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139.
- Kalinina, N.I.; Sysoeva, V.Y.; Rubina, K.A.; Parfenova, Y.V.; Tkachuk, V.A. Mesenchymal stem cells in tissue growth and repair. Acta Nat. 2011, 3, 30–37.
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Pavlova, G.; Parfyonova, Y.; Tkachuk, V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE 2011, 6, e17899.
This entry is offline, you can click here to edit this entry!
