Smoking cessation interventions are effective, but they are not easily accessible for all treatment-seeking smokers. Mobile health (mHealth) apps have been used in recent years to overcome some of these limitations. Smoking cessation apps can be used in combination with a face-to-face intervention (FFSC-Apps), or alone as general apps (GSC-Apps). Smartphone apps for smoking cessation could be promising tools. However, more research with an adequate methodological quality is needed to determine its effect. Nevertheless, smartphone apps’ high availability and attractiveness represent a great opportunity to reach large populations.
- smoking cessation
- mHealth
- smartphone
- mobile phone
- digital health
1. Introduction
2. Smoking Cessation Apps
2.1 Effects of Smartphone Apps on Abstinence, Tobacco Use, and Relapse Rates
2.1.1. General Apps for Smoking Cessation (GSC-Apps)
Concerning smoking cessation outcomes (Table 1), two studies found significant differences in abstinence rates between conditions at each point-assessment [17][18] and one at the 6-months but not at the 3-months point-assessment [19]. The remaining two studies reported similar quit rates between the experimental and the control group at each point-assessment [20][21]. Regarding cigarettes per day (CPD) outcomes, one study found significant differences between conditions [22] and two studies did not find significant differences [21][19].
| General Smartphone Apps | |||
|---|---|---|---|
| Author | Abstinence | Tobacco Use | Relapse Rates |
| Baskerville et al. (2018) [19] | Significant differences between conditions at 6 months point-assessment in favor of the control group (22.3% vs. 18.3%). | Nonsignificant differences in CPD at 6-month point-assessment between conditions. | Not reported. |
| BinDhim et al. (2018) [17] | Significant differences between conditions in continuous abstinence rates at 10-days (32.2% vs. 20.8%), 1- (28.5% vs. 16.9%), 3- (23.8% vs. 10.2), and 6-month point-assessments (10.2% vs. 4.8%) in favor of the experimental group. | Not reported. | Not reported. |
| Bricker et al. (2014) [20] | Nonsignificant differences between conditions at 2-month post-enrollment point-assessment (13% experimental vs. 8% control). | Not reported. | Not reported. |
| Bricker et al. (2017) [23] | 21% for 7-day PPA and 11% for 30-day PPA at 2-month-post-enrollment point-assessment. | 75% reduction rate of CPD at 2-month point-assessment. | Not reported. |
| Buller et al. (2014) [18] | Significant differences between conditions at 6-week point-assessment in favor of the control group (58% vs. 30%). | Not reported. | Not reported. |
| Dar (2017) [22] | Not reported. | Significant differences in CPD reduction between conditions in favor of the experimental group at the end of the study. | Not reported. |
| Garrison et al. (2020) [21] | Nonsignificant differences between conditions at 6-month point-assessment (9.8% experimental vs. 12.1% control). | Nonsignificant differences between conditions in CPD reduction. Significant reductions in CPD from baseline to the 6-month point-assessment. |
Not reported. |
| Iacoviello et al. (2017) [24] | 45.2% for 7-day PPA and 26.2% for 30-day PPA at the end of the study. | Not reported. | Not reported. |
| Marler et al. (2019) [25] | 32.0% for 7-day PPA and 27.6% for 30-day PPA at the end of the study. | Nonabstinent participants reduced 29.1% in CPD at the end of the study. | Not reported. |
| Combine apps with face-to-face contact | |||
| Author | Abstinence | Tobacco use | Relapse rates |
| Businelle et al. (2016) [26] | 41% at quit date, 17% at 1-week, 31% at 2-week, 27% at 3-week, 22% at 4-week, and 20% at 12-week point-assessment. | Not reported. | Not reported. |
| Carpenter et al. (2015) [27] | 50% at 4 weeks. Of these, 65% at 3-months and 60% at 6-months point-assessment remained abstinent. | Not reported. | Not reported. |
| Dan et al. (2016) [28] | 3% at baseline, 42% at tapering, 55% at treatment, and 42% at thinning. 0% at 1-week follow-up were abstinent. |
Not reported. | Not reported. |
| Hébert et al. (2020) [29] | Nonsignificant differences between conditions. 22% Smart-T2, 26% QuitGuide, 30% usual care at 4 weeks point-assessment. 22% Smart-T2, 15% QuitGuide, 15% usual care at 12-weeks point-assessment. |
Not reported. | Not reported. |
| Hertzberg et al. (2013) [30] | Nonsignificant differences between conditions at 4-week point-assessment (82% experimental vs. 45% control). | Not reported. | Not reported. |
| Hicks et al. (2017) [31] | Nonsignificant differences between conditions at post-treatment (60% experimental vs. 100% control) and at 2-week point-assessment (60% experimental vs. 67% control). | Not reported. | Not reported. |
| Janes et al. (2019) [32] | Not reported. | Nonsignificant differences between conditions in CPD reduction. Significant reductions in CPD from baseline to 1-month follow-up under both conditions. |
Not reported. |
| Krishnan et al. (2019) [33] | Nonsignificant differences between conditions at 30-day point-assessment (3% experimental vs. 2% control). | Nonsignificant differences in CPD between baseline and 30-day point-assessment. | Not reported. |
| Masaki et al. (2019) [34] | 64% from weeks 9 to 24, 76% from weeks 9 to 12, and 58% from 9 to 52 weeks in continuous abstinence rate. | Not reported. | Not reported. |
| Masaki et al. (2020) [35] | Significant differences between conditions in continuous abstinence rates from weeks 9 to 12 (75.4% vs. 66.2%), 9 to 24 (63.9% vs. 50.5%), and 9 to 52 (52.3% vs. 41.5%) in favor of the experimental group. | Not reported. | Significant differences between conditions in time to the first lapse after the quit date in favor of the experimental group. |
| McClure et al. (2018) [36] | 25% at the quit date and 0% at the 5-day follow-up. | Not reported. | Not reported. |
| Minami et al. (2018) [37] | 12.5% at 2-week, 4-week, and 3-months point-assessment. | All participants reported reductions in CPD from baseline to 2-week, 4-week, and 3-month point-assessments. | Not reported. |
| O’Connor et al. (2020) [38] | Nonsignificant differences between conditions at post-treatment (36% combined group, 20% ACT, and 24% behavioral support). Nonsignificant differences between conditions at 6-month follow-up (24% combined group, 24% ACT, and 20% behavioral support). |
Significant differences in CPD reduction in favor of the combined condition. | Not reported. |
| Raiff et al. (2017) [39] | 1.25% at baseline, 13.8% at tapering, 35.5% at abstinence induction, and 0% at 1-month follow-up. | Not reported. | Not reported. |
| Wilson et al. (2019) [40] | Cohort 1: 40% at post-treatment and 20% at 3-months follow-up. Cohort 2: 38% at post-treatment and 15% at 3-months follow-up. |
Cohort 1: 20% reduced CPD at post-treatment. Cohort 2: 38% reduced CPD at post-treatment |
Not reported. |
2.1.2. Combine Apps with Face-to-Face Contact (FFSC-Apps)
Of the seven studies that used comparison groups, four were RCTs [29][38][35][32] and three were CCTs [30][31][33]. Four studies compared two mobile apps [30][31][35][32], one compared the use of a mobile app (experimental group) to brief advice (control group) [33], and two studies compared three treatment conditions [29][38]. Regarding smoking cessation outcomes (Table 1), one showed significant differences in abstinence rates between groups [35]. Regarding CPD outcomes, one study found significant reductions in CPD from baseline to the 1-month follow-up [32], and one study showed that participants who had not stopped smoking in the combined condition (app combined with Acceptance and Commitment Therapy (ACT) face-to-face treatment) reported significantly less CPD at post-treatment compared to the other two conditions. Finally, one study found no significant differences between study arms [33]. The remaining studies did not report CPD outcomes.
2.2. Effects of Smartphone Apps on Abstinence, Tobacco Use, and Relapse Rates Regarding the Methodological Quality of the Studies
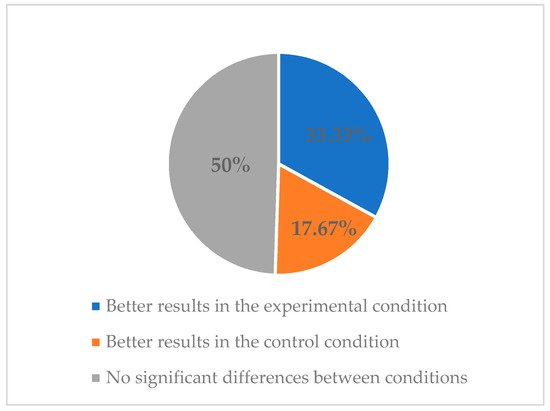
2.3. Features of Smartphone Apps for Smoking Cessation
Regarding the features of smoking cessation apps (Table 3), we clustered them into the following groups depending on the content of the apps: CO; set a quit date; EMAS; self-tracking or smoking self-report; mindfulness content; and ACT content.
3. Summary
Overall, smoking cessation apps are useful tools for smoking cessation. Most studies using a comparison group showed that smartphone apps were at least as useful as the control conditions (e.g., brief advice, other mobile apps), obtaining abstinence rates at the end of treatment ranging from 36 to 100%. Regarding before-and-after studies, the abstinence rates obtained ranged between 12.5 and 51.5%. Despite these abstinence outcomes being lower than those obtained in conventional psychological and pharmacological interventions [41], the possibility of increasing treatment access to a wider population of smokers makes them promising tools in terms of public health impact. Additionally, results from studies measuring CPD suggest that smoking cessation apps are also as effective as control groups (e.g., print-based self-help materials, other mobile apps) in reducing cigarette use. More research is needed to obtain more accurate conclusions about relapse rates, because only one study assessed this outcome.
In summary, smoking cessation apps are promising tools that could be easily integrated into smoking cessation treatments. They may be able to improve some clinical aspects such as motivation and treatment adherence. Moreover, professionals can use these apps to facilitate communication with the patient, provide content in an easier way, and obtain different data that can improve the effectiveness of treatments.
More research with strong methodological quality is needed to determine more accurately the effect of mobile apps, combined or not with face-to-face contact, on smoking cessation outcomes. Moreover, future studies should design smoking cessation apps adhering to standard guidelines [42][43] and using rigorous methodologies, including sample size calculations, intention-to-treat analysis, and longer follow-up periods. Due to the emerging development of this field, it is expected that future research will resolve the current limitations to draw clear conclusions.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph182111664
References
- Centers for Disease Control and Prevention (CDC). Current Cigarette Smoking Among Adults in the United States. 2021. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed on 1 March 2021).
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous Compounds in tobacco smoke. Int. J. Environ. Res. Public. Health 2011, 8, 613–628.
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic, 2019: Offer Help to Quit Tobacco Use. 2020. Available online: https://apps.who.int/iris/handle/10665/326043 (accessed on 1 March 2021).
- Goldenberg, M.; Danovitch, I.; IsHak, W.W. Quality of life and smoking. Am. J. Addict. 2014, 23, 540–562.
- Prochaska, J.J.; Das, S.; Young-Wolff, K.C. Smoking, Mental Illness, and Public Health. Annu. Rev. Public Health 2017, 38, 165–185.
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafò, M.R. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. 2017, 19, 3–13.
- Siu, A.L. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2015, 163, 622–634.
- West, R.; Stapleton, J. Clinical and public health significance of treatments to aid smoking cessation. Eur. Respir. Rev. 2008, 17, 199–204.
- García, M.C.; Bastian, B.; Rossen, L.M.; Anderson, R.; Miniño, A.; Yoon, P.W.; Faul, M.; Massetti, G.; Thomas, C.C.; Hong, Y.; et al. Potentially Preventable Deaths Among the Five Leading Causes of Death—United States, 2010 and 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1245–1255.
- Fiore, M.C.; Jaén, C.R.; Baker, T.B.; Bailey, W.C.; Benowitz, N.L.; Curry, S.J.; Dorfman, S.F.; Froelicher, E.S.; Goldstein, M.G.; Healton, C.G.; et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. 2008. Available online: https://www.ahrq.gov/prevention/guidelines/tobacco/index.html (accessed on 1 March 2021).
- World Health Organization (WHO). Global Diffusion of EHealth: Making Universal Health Coverage Achievable. Report of the Third Global Survey on EHealth. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/252529/9789241511780-eng.pdf;jsessionid=0FDF0200A88173BE06F79C193B8E60DA?sequence=1 (accessed on 1 March 2021).
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y.; Dobson, R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, 10, CD006611.
- Keoleian, V.; Polcin, D.; Galloway, G.P. Text messaging for addiction: A review. J. Psychoact. Drugs 2015, 47, 158–176.
- Spohr, S.A.; Nandy, R.; Gandhiraj, D.; Vemulapalli, A.; Anne, S.; Walters, S.T. Efficacy of SMS Text Message Interventions for Smoking Cessation: A Meta-Analysis. J. Subst. Abuse Treat. 2015, 56, 1–10.
- Vilardaga, R.; Casellas-Pujol, E.; McClernon, J.F.; Garrison, K.A. Mobile Applications for the Treatment of Tobacco Use and Dependence. Curr. Addict. Rep. 2019, 6, 86–97.
- BinDhim, N.F.; McGeechan, K.; Trevena, L. Smartphone Smoking Cessation Application (SSC App) trial: A multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid “app”. BMJ Open 2018, 8, e017105.
- Buller, D.B.; Borland, R.; Bettinghaus, E.P.; Shane, J.H.; Zimmerman, D.E. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed. e-Health 2014, 20, 206–214.
- Baskerville, N.B.; Struik, L.L.; Guindon, G.E.; Norman, C.D.; Whittaker, R.; Burns, C.; Hammond, D.; Dash, D.; Brown, K.S. Effect of a Mobile Phone Intervention on Quitting Smoking in a Young Adult Population of Smokers: Randomized Controlled Trial. JMIR MHealth UHealth 2018, 6, e10893.
- Bricker, J.B.; Mull, K.E.; Kientz, J.A.; Vilardaga, R.; Mercer, L.D.; Akioka, K.J.; Heffner, J.L. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014, 143, 87–94.
- Garrison, K.A.; Pal, P.; O’Malley, S.S.; Pittman, B.P.; Gueorguieva, R.; Rojiani, R.; Scheinost, D.; Dallery, J.; Brewer, J.A. Craving to Quit: A Randomized Controlled Trial of Smartphone App-Based Mindfulness Training for Smoking Cessation. Nicotine Tob. Res. 2020, 22, 324–331.
- Dar, R. Effect of Real-Time Monitoring and Notification of Smoking Episodes on Smoking Reduction: A Pilot Study of a Novel Smoking Cessation App. Nicotine Tob. Res. 2018, 20, 1515–1518.
- Bricker, J.B.; Copeland, W.; Mull, K.E.; Zeng, E.Y.; Watson, N.L.; Akioka, K.J.; Heffner, J.L. Single-arm trial of the second version of an acceptance & commitment therapy smartphone application for smoking cessation. Drug Alcohol Depend. 2017, 170, 37–42.
- Iacoviello, B.M.; Steinerman, J.R.; Klein, D.B.; Silver, T.L.; Berger, A.G.; Luo, S.X.; Schork, N.J. Clickotine, A Personalized Smartphone App for Smoking Cessation: Initial Evaluation. JMIR MHealth UHealth 2017, 5, e56.
- Marler, J.D.; Fujii, C.A.; Utley, D.S.; Tesfamariam, L.J.; Galanko, J.A.; Patrick, H. Initial Assessment of a Comprehensive Digital Smoking Cessation Program that Incorporates a Mobile App, Breath Sensor, and Coaching: Cohort Study. JMIR MHealth UHealth 2019, 7, e12609.
- Businelle, M.S.; Ma, P.; Kendzor, D.E.; Frank, S.G.; Vidrine, D.J.; Wetter, D.W. An Ecological Momentary Intervention for Smoking Cessation: Evaluation of Feasibility and Effectiveness. J. Med. Internet Res. 2016, 18, e321.
- Carpenter, V.L.; Hertzberg, J.S.; Kirby, A.C.; Calhoun, P.S.; Moore, S.D.; Dennis, M.F.; Dennis, P.A.; Dedert, E.A.; Hair, L.P.; Beckham, J.C. Multicomponent smoking cessation treatment including mobile contingency management in homeless veterans. J. Clin. Psychiatry 2015, 76, 959–964.
- Dan, M.; Grabinski, M.J.; Raiff, B.R. Smartphone-based contingency management for smoking cessation with smokers diagnosed with attention-deficit/hyperactivity disorder. Transl. Issues Psychol. Sci. 2016, 2, 116–127.
- Hébert, E.T.; Ra, C.K.; Alexander, A.C.; Helt, A.; Moisiuc, R.; Kendzor, D.E.; Vidrine, D.J.; Funk-Lawler, R.K.; Businelle, M.S. A Mobile Just-in-Time Adaptive Intervention for Smoking Cessation: Pilot Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e16907.
- Hertzberg, J.S.; Carpenter, V.L.; Kirby, A.C.; Calhoun, P.S.; Moore, S.D.; Dennis, M.F.; Dennis, P.A.; Dedert, E.A.; Beckham, J.C. Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine Tob. Res. 2013, 15, 1934–1938.
- Hicks, T.A.; Thomas, S.P.; Wilson, S.M.; Calhoun, P.S.; Kuhn, E.R.; Beckham, J.C. A preliminary investigation of a relapse prevention mobile application to maintain smoking abstinence among individuals with posttraumatic stress disorder. J. Dual Diagn. 2017, 13, 15–20.
- Janes, A.C.; Datko, M.; Roy, A.; Barton, B.; Druker, S.; Neal, C.; Ohashi, K.; Benoit, H.; van Lutterveld, R.; Brewer, J.A. Quitting starts in the brain: A randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychopharmacology 2019, 44, 1631–1638.
- Krishnan, N.; Elf, J.L.; Chon, S.; Golub, J.E. COach2Quit: A Pilot Randomized Controlled Trial of a Personal Carbon Monoxide Monitor for Smoking Cessation. Nicotine Tob. Res. 2019, 21, 1573–1577.
- Masaki, K.; Tateno, H.; Kameyama, N.; Morino, E.; Watanabe, R.; Sekine, K.; Ono, T.; Satake, K.; Suzuki, S.; Nomura, A.; et al. Impact of a Novel Smartphone App (CureApp Smoking Cessation) on Nicotine Dependence: Prospective Single-Arm Interventional Pilot Study. JMIR MHealth UHealth 2019, 7, e12694.
- Masaki, K.; Tateno, H.; Nomura, A.; Muto, T.; Suzuki, S.; Satake, K.; Hida, E.; Fukunaga, K. A Randomized Controlled Trial of a Smoking Cessation Smartphone Application with a Carbon Monoxide Checker. Npj Digit. Med. 2020, 3, 35.
- McClure, E.A.; Tomko, R.L.; Carpenter, M.J.; Treiber, F.A.; Gray, K.M. Acceptability and compliance with a remote monitoring system to track smoking and abstinence among young smokers. Am. J. Drug Alcohol Abuse 2018, 44, 561–570.
- Minami, H.; Brinkman, H.R.; Nahvi, S.; Arnsten, J.H.; Rivera-Mindt, M.; Wetter, D.W.; Bloom, E.L.; Price, L.H.; Vieira, C.; Donnelly, R.; et al. Rationale, design and pilot feasibility results of a smartphone-assisted, mindfulness-based intervention for smokers with mood disorders: Project mSMART MIND. Contemp. Clin. Trials 2018, 66, 36–44.
- O’Connor, M.; Whelan, R.; Bricker, J.; McHugh, L. Randomized Controlled Trial of a Smartphone Application as an Adjunct to Acceptance and Commitment Therapy for Smoking Cessation. Behav. Ther. 2020, 51, 162–177.
- Raiff, B.R.; Arena, A.; Meredith, S.E.; Grabinksi, M.J. Feasibility of a mobile group financial-incentives intervention among pairs of smokers with a prior social relationship. Psychol. Rec. 2017, 67, 231–239.
- Wilson, S.M.; Thompson, A.C.; Currence, E.D.; Thomas, S.P.; Dedert, E.A.; Kirby, A.C.; Elbogen, E.B.; Moore, S.D.; Calhoun, P.S.; Beckham, J.C. Patient-informed treatment development of behavioral smoking cessation for people with schizophrenia. Behav. Ther. 2019, 50, 395–409.
- Patnode, C.D.; Henderson, J.T.; Melnikow, J.; Coppola, E.L.; Durbin, S.; Thomas, R. Interventions for Tobacco Cessation in Adults, Including Pregnant Women: An Evidence Update for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2021; pp. 1–266.
- Chatzipavlou, I.A.; Christoforidou, S.A.; Vlachopoulou, M. A recommended guideline for the development of mHealth Apps. mHealth 2016, 2, 21.
- Llorens-Vernet, P.; Miró, J. Standards for Mobile Health–Related Apps: Systematic Review and Development of a Guide. JMIR MHealth UHealth 2020, 8, e13057.
