The heart is a metabolic omnivore that combusts a considerable amount of energy substrates, mainly long-chain fatty acids (FAs) and others such as glucose, lactate, ketone bodies, and amino acids. There is emerging evidence that muscle-type continuous capillaries comprise the rate-limiting barrier that regulates FA uptake into cardiomyocytes. The transport of FAs across the capillary endothelium is composed of three major steps—the lipolysis of triglyceride on the luminal side of the endothelium, FA uptake by the plasma membrane, and intracellular FA transport by cytosolic proteins. In the heart, impaired trans-endothelial FA (TEFA) transport causes reduced FA uptake, with a compensatory increase in glucose use. In most cases, mice with reduced FA uptake exhibit preserved cardiac function under unstressed conditions. When the workload is increased, however, the total energy supply relative to its demand (estimated with pool size in the tricarboxylic acid (TCA) cycle) is significantly diminished, resulting in contractile dysfunction. The supplementation of alternative fuels, such as medium-chain FAs and ketone bodies, at least partially restores contractile dysfunction, indicating that energy insufficiency due to reduced FA supply is the predominant cause of cardiac dysfunction.
- cardiac metabolism
- fatty acid
- capillary endothelium
- trans-endothelial fatty acid transport
- contractile function
1. Mechanisms of FA Uptake by the Heart
1.1. Source of Long-Chain Fatty Acids
1.2. Lipolysis of TG Contained in TG-Rich Lipoproteins on the Luminal Side of the Capillary Endothelium
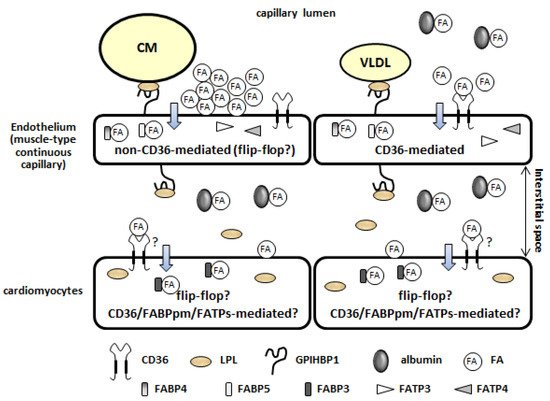
1.3. Fatty Acid Uptake by the Plasma Membrane of the Capillary Endothelium (Non-CD36-Mediated and CD36-Mediated Pathways)
1.4. Intracellular Fatty Acid Transport through the Capillary Endothelium
1.5. Fatty Acid Uptake by Cardiomyocytes
2. Molecular Mechanisms Underlying the Induction of Genes Associated with Trans-Endothelial Fatty Acid Transport
Recent studies have revealed that the expression of genes associated with TEFA transport is regulated by several ligands, receptors, and transcription factors (Table 1) [6,7,8,9]. It is likely that these systems can be roughly divided into two groups according to their target genes. One includes the peroxisome proliferator-activated receptor γ (PPARγ), mesodermal homeobox-2/transcription factor 15 (Meox2/Tcf15), Notch signaling, and the apelin/apelin receptor (APLNR), and it mainly controls the expression of CD36, FABPs, and GPIHBP1. The other is a group that includes the VEGF-B/VEGF receptor (VEGFR), angiopoietin-like 2 (ANGPTL2), and 3-hydroxyisobutyrate (3-HIB), and it regulates the expression/function of FATP3/4 (Table 1). Although impairments of the systems influence both local and systemic metabolism, cardiac metabolism seems to only be affected by PPARγ, Meox2/Tcf15, Notch signaling, and VEGF-B/VEGFR (Table 1) [6,7,8,9]. The trans-endothelial transport of other substrates and molecules (e.g., lipoproteins, lipoprotein lipase, glucose, and insulin) and endothelium-derived metabolic regulators (e.g., nitric oxide, extracellular matrix proteins, hormones, growth factors, and enzymes) is described elsewhere [6,7,9].
| Ligand | Receptor/Transcription Factor | Target Genes | Target Tissues Influenced by the System | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPARγ | CD36 | FABP4 | FABP5 | LPL | GPIHBP1 | ANGPTL4 | LIPG | FATP3 | FATP4 | ||||
| PPARγ | ⚪ | ⚪ | ⚪ | heart, skeletal muscle, adipose tissue | [35,36,37] | ||||||||
| Meox2/Tcf15 | ⚪ | ⚪ | ⚪ | ⚪ | ⚪ | ⚪ | heart | [38] | |||||
| Dll4 | Notch1/N1-ICD/Rbp-jκ | independent | ⚪ | ⚪ | ⚪ | ⚫ | ⚪ | heart, skeletal muscle | [39,40] | ||||
| Apelin | APLNR/phosphorylation of FOXO1 | ⚫ | skeletal muscle | [41] | |||||||||
| VEGF-B | VEGFR/NPR1 | ⚪ | ⚪ | heart, BAT, skeletal muscle | [26] | ||||||||
| ANGPTL2 | integrin α5β1 | ⚪ | ⚪ | subcutaneous adipose tissue | [42] | ||||||||
| 3-HIB | ⚪* | ⚪* | skeletal muscle | [43] | |||||||||
2.1. Peroxisome Proliferator-Activated Receptor γ
2.2. Mesodermal Homeobox-2/Transcription Factor 15
2.3. Notch Signaling
| Target Genes | Deficient Site | Inducible Knockout | VLDL-TG Uptake | FA Uptake | Glucose Uptake | Glut1/4 | Ketonein Serum | Contractile Performance In Vivo Estimated by Echocardiography | Reference |
|---|---|---|---|---|---|---|---|---|---|
| LPL (functions at luminal side of capillary) | cardiomyocyte | ↓ | ↑ | ↑ | ↑ | ↓ aged | [44] | ||
| cardiomyocyte | ⚪ | ↓ | [45] | ||||||
| CD36 | whole | ↓ | ↑ | ↑ | ↑ | intact | [46,47,48,49] | ||
| whole | ↓ | ↑ | prevention from age-induced cardiomyopathy | [49] | |||||
| endothelium | ↓ | ↑ | ↑ | not available | [21] | ||||
| FABP4/5 | whole | ↓ | ↑ | ↑ | ↑ | intact | [23,50] | ||
| Meox2+/−:Tcf15+/− | endothelium: whole | ↓ | ↑ | ↓ aged | [38] | ||||
| Rbp-jκ (Notch signal) | endothelium | ⚪ | ↓ | ↑ | ↓ | ↓↓ | [39] | ||
| PPARγ | endothelium | →↓ | → | intact (personal observation) | [35] | ||||
| VEGF-B | whole | ↓ | ↑ | ↑ | not available | [26] | |||
| FABP3 | whole | ↓ | ↑ | → | ↑ | not available | [51,52] | ||
| CD36 | cardiomyocyte | → | → | not available | [21] | ||||
| cardiomyocyte | ⚪ | ↓ (ex vivo) | ↑ (ex vivo) | intact | [53,54] |
2.4. Apelin/Apelin Receptor/Forkhead Box O1
2.6. Angiopoietin-Like 2/Integrin α5β1
2.7. 3-Hydroxyisobutyrate
3. Association between In Vivo Cardiac Metabolism and Contractile Function in Mice with Reduced Fatty Acid Uptake
3.1. Limitation of Experiments with Ex Vivo Perfused Hearts
3.2. In Vivo Cardiac Metabolism and Contractile Function in Mice with Reduced Trans-Endothelial Fatty Acid Transport under Unstressed Conditions
3.3. In Vivo Cardiac Metabolism in CD36 KO Mice under Unstressed Conditions
3.4. In Vivo Contractile Dysfunction in Mice with Reduced Trans-Endothelial Fatty Acid Transport under an Increased Afterload
3.5. Pool Size in the TCA Cycle as a Useful Marker for Energy Status
3.6. Mechanism Underlying the Enhancement of Glycolytic Flux in the Hearts of Mice with Reduced Fatty Acid Uptake
This entry is adapted from the peer-reviewed paper 10.3390/metabo11120889
