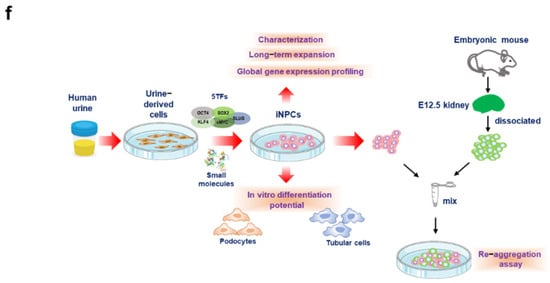
Chronic kidney disease (CKD) has emerged as a major public health concern due to its prevalence in 7–12% of the population worldwide, progression to irreversible end-stage renal disease (ESRD), impaired quality of life, associations with high social and financial costs, and high rates of associated morbidity and mortality (an 82% increase in CKD epidemic over the past two decades). The current treatment options for kidney failure involve lifelong dialysis and whole kidney transplantation. Although kidney transplantation undoubtedly offers a better quality of life and life expectancy than dialytic treatment, it is limited by the scarcity of available organs and the huge gap between supply and demand. Furthermore, considering that the average life expectancy of dialysis patients is barely a decade, alternative strategies for preventing or delaying the progression to ESRD are urgently needed. In this context, regenerative medicine strategies employing nephron progenitor cells (NPCs) are a viable approach that is worthy of substantial consideration as a promising cell source for kidney diseases. However, the generation of induced nephron progenitor-like cells (iNPCs) from human somatic cells remains a major challenge. Here, we describe a novel method for generating NPCs from human urine-derived cells (UCs) that can undergo long-term expansion in a serum-free condition. Here, we generated iNPCs from human urine-derived cells by forced expression of the transcription factors OCT4, SOX2, KLF4, c-MYC, and SLUG, followed by exposure to a cocktail of defined small molecules. These iNPCs resembled human embryonic stem cell-derived NPCs in terms of their morphology, biological characteristics, differentiation potential, and global gene expression and underwent a long-term expansion in serum-free conditions. As a results, this study demonstrates that human iNPCs can be readily generated and expanded, which will facilitate their broad applicability in a rapid, efficient, and patient-specific manner, particularly holding the potential as a transplantable cell source for patients with kidney disease.
- nephron progenitor cells
- direct reprogramming
- transdifferentiation
- urine cells
- kidney
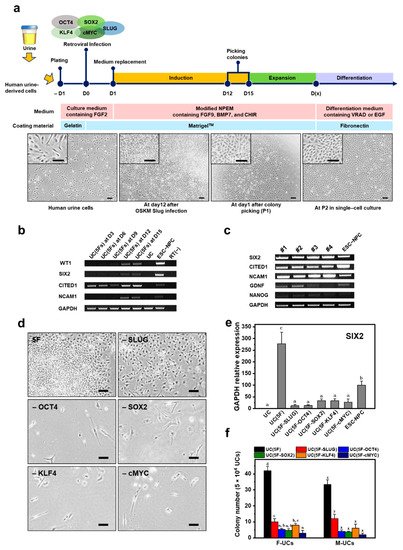
1. Screening for NPC-Inducing Factors
2. Generation of iNPCs from Human UCs
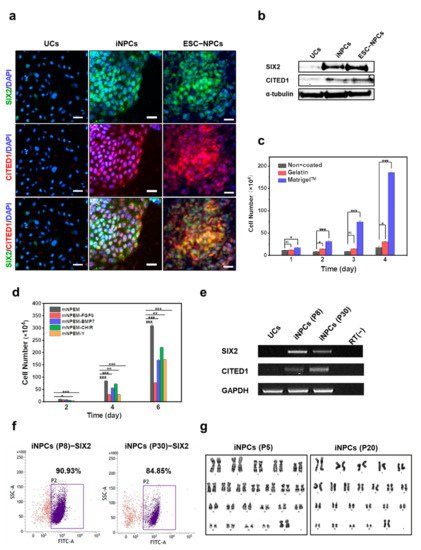
3. Characterization and In Vitro Expansion of iNPCs
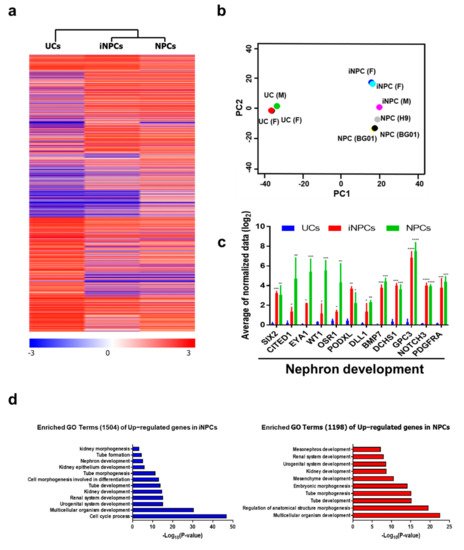
4. Global Gene Expression Analysis of iNPCs
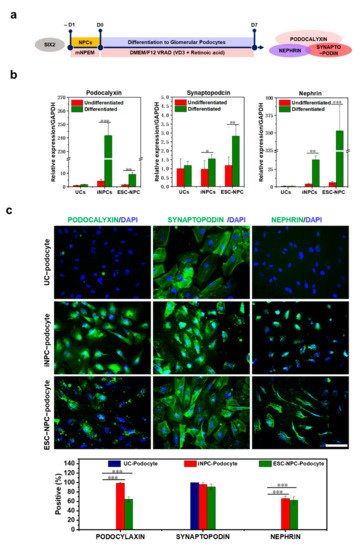
5. Differentiation Potential of iNPCs
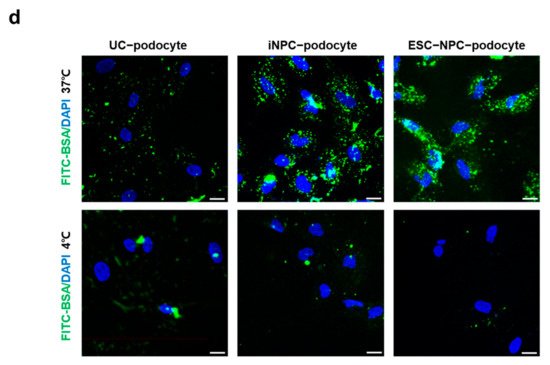
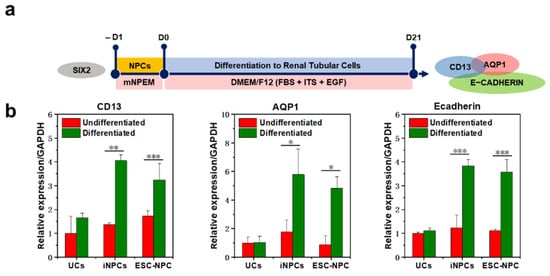
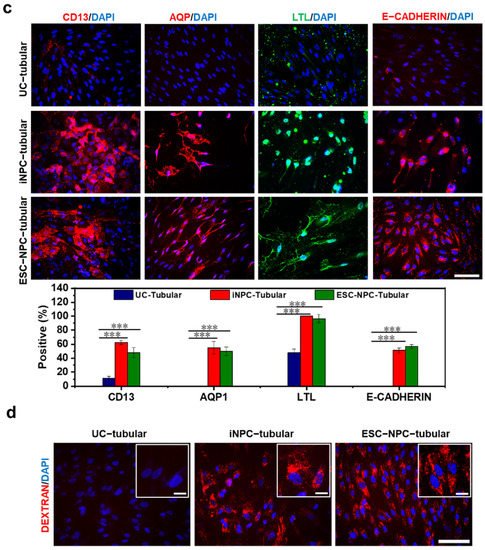
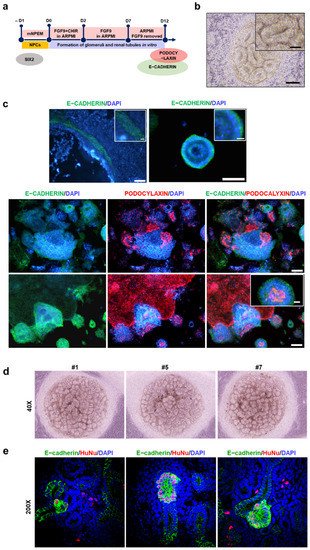
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413449
