Collagen is a natural polymer found abundantly in the extracellular matrix (ECM). It is easily extracted from a variety of sources and exhibits excellent biological properties such as biocompatibility and weak antigenicity. Additionally, different processes allow control of physical and chemical properties such as mechanical stiffness, viscosity and biodegradability. Moreover, various additive biomanufacturing technology has enabled layer-by-layer construction of complex structures to support biological function. Additive biomanufacturing has expanded the use of collagen biomaterial in various regenerative medicine and disease modelling application (e.g., skin, bone and cornea).
1. Introduction
Collagen is by far the most prevalent extracellular matrix (ECM) molecule found in adult mammals with an estimated 30% of protein mass of multicellular organisms [
1]. Although the collagen molecule has 29 subtypes (variants) [
2,
3], approximately 90% of collagen consists of variants types I, II, III [
4]. Collagen extracellular matrix can be found throughout the body in both soft and hard connective tissues including bones, skin, tendon, cartilage, cornea, lung, liver etc. [
5].
Its fundamental structural unit is a 300 nm protein consisting of 3 braided α-subunits of 1050 amino acids in length. Each strand comprises the repeating amino acid motif: Gly-Pro-X (X is any amino acid). These strands form hydrogen bonds between the NH bond of a glycine and a carbonyl (C=O) group from an adjacent strand that holds the structure together and form their characteristic triple helix structure [
4,
6]. Collagen is a hierarchical biomaterial that is self-assembled into fibrils (containing numerous structural units) of ~1 cm length and ~500 nm in diameter (using type 1 Collagen as the archetype). Fascinatingly, the individual tropocollagen monomers are unstable at body temperature and favour random coil conformations. However, collagen fibrillogenesis gives rise to triple helix macromolecular structures with favourable mechanical strength in 3-dimensions, with resistance to enzymatic degradation [
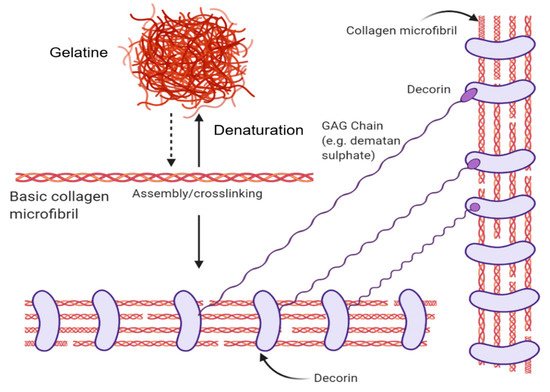
6]. Through the introduction of energy (e.g., heat energy from the surroundings), the H-bonds maintaining the orderly collagen structure are separated, causing the individual strands of the triple-helix to separate, resulting in a disorganized, denatured state known as gelatin (please see
Figure 1 for more information).
Figure 1. The structural forms of collagen and their native interactions. The basic collagen unit is a triple-helix microfiber that denatures into gelatine or can be assembled into collagen fibrils. Decorin proteins wrap around collagen fibrils in their native context and bind with glycosaminoglycan chains such as dermatan sulphate. Created with BioRender.com.
The Gly-Pro-X amino acid arrangement is critical to the collagen molecule as seen from disease-causing mutations that lead to osteogenesis imperfecta or “brittle bone” disease. A single misplacement of glycine due to the mutation results in unstable helices [
4]. In their native microenvironment, collagen molecules interact with other biological molecules. Negatively-charged Glycosaminoglycans (linear polysaccharides) sequester growth factors within the ECM [
7]. These have been used to generate bio-active collagen scaffolds for cell growth [
8]. Furthermore, Collagen interacts with Elastin fibers to provide recoil to the ECM, as well as fibronectin to mediate cell attachment and function [
1]. Collagen molecules can also interact with reducing sugars in the body which result in its glycation. Glycation molecules result in the formation of advanced glycation end products (AGEs) which gives rise to the loss of soft tissue biomechanical properties and is associated with various diseases such as atherosclerosis, osteoporosis, diabetes and renal failure [
9].
Collagen biomaterials have been utilised for decades to enhance cell culture/function [
10]. A number of collagen or collagen-derivative based protocols and commercial culture products have been used extensively ranging from cell culture surfaces to hydrogels [
10]. These include culture well inserts [
11,
12] (MilliCell
®, Transwell
®), sponge/gels (Matrigel™, Extracel™) and microcarriers (GEM™). While matrigel is derived from Engelbreth–Holm–Swarm (EHS) tumor and found to contain collagen IV, laminin and heparin sulfate, GEM ™ microcarriers coat an alginate core with gelatin to aid cell attachment.
Beyond cell culture reagents, collagen biomaterials have been used for tissue engineering applications including: bone, tendon, cardiovascular therapies and disease models [
13], cornea [
5], skin, skeletal muscle, artery [
14] etc. One usage with great popularity is using collagen scaffolds as dermal regeneration templates for severe wounds and other trauma such as burns. To date, a number of scaffolds/templates containing collagen ingredients are commercially available including: Helistat (Integra
®), Instat (Johnson & Johnson), SkinTemp (BioCor), Helitene (Integra
®), Fibracol (J&J), Biobrane (UDL Laboratories), and Chronicure (Derma Sciences)–not an exhaustive list, which is currently presented in fibre, powder, composite forms etc. [
15]. Collagen biomaterials as dermal templates have seen the greatest number of commercial translations to date. Recently, novel applications in sustainable cellular agriculture using collagen biomaterials include making artificial leather and bio-artificial muscle [
16].
Despite plentiful collagen biomaterial applications developed, collagen has several limitations that curtail its widespread usage: generally poor mechanical properties (vascular tissue engineering applications), thrombogenicity, contamination, source and batch variability [
13]. These limitations leave many collagen biomaterial applications in the earlier technology development stages, hindering technology translation.
The emerging field of biomaterials printing - bioprinting, provides the means to create structures from collagen biomaterials, additives and cells in a reproducible and scalable way [
17,
18]. Adapted from methods first used to manufacture inorganic materials [
19], bioprinting is an additive manufacturing approach to produce living tissue and organ analogs for regenerative medicine, tissue engineering, pharmacokinetic and disease/developmental modelling [
20]. By patterning various combinations of biomaterials and cells, a goal is to reproduce complex biological architecture to recreate the anatomy in reproducible ways [
21,
22]. Thus, bioprinting potentially mitigates concerns of product variability by increasing process reproducibility. Moreover, increasing production throughput with bioprinting circumvents bottlenecks in production capacity, making collagen biomaterial products more cost-effective.
2. Processing Parameters
Each step in the processing of collagen for additive manufacturing alters the properties and structure of collagen. Depending on the sources of collagen, extraction steps and crosslinking methods (chemical, physical), the resultant properties will differ. The effects of these processes as well as methods for analyzing collagen biomaterials will be discussed.
2.1. Sources of Collagen
For additive biomanufacturing, fibril-forming sub-types of collagen (type I, type II, type III, type V, type XI, type XXIV and type XXVII) are preferred because they contribute to the mechanical integrity of the ECM [
15,
24]. Fibrillar collagen is formed from the assembly of collagen molecules because of the intermolecular bonds between the individual strands to create the signature triple-helix collagen molecule (see the introduction section). These fibrils further assemble into fibre-bundles with tensile strength in tendons and skin [
3] or into orthogonal transparent layers (e.g., cornea) [
25].
Fibrillar collagen can be extracted from various sources. As animal skin/tendons and cartilaginous tissues are abundant in type I and type II collagen respectively, these tissues are sources of fibrillar collagen extraction [
26]. Cells cultured in vitro are used to synthesize collagen as well [
27,
28]. Cells such as fibroblast and chondrocytes which specialize in type I and type II collagen production respectively can be cultured and the synthesised collagen harvested from media or cell layers. Recombinant collagen production is using genetically engineered microorganisms, plants or animals such as bacteria, yeast, transgenic corn and silkworms [
29,
30]. Synthetic peptides mimicking collagen trimeric structure have also been investigated to produce collagen-like peptides [
31,
32]. Collagen from cells grown in vitro, recombinant protein production as well as peptide synthesis have very low yield and are not as cost-effective as collagen extraction from animal tissues. Hence, most commercial collagen extraction relies on animal sources. While there are variations in collagen between different animal species and tissue sources, variation of collagen exists as well, within the same species due to the nature of collagen. As the collagen molecules in animals form mature crosslinks over time, the age, gender, activity and physical state of the animals play a significant role in forming these crosslinks [
2]. The variability of collagen between batches of extraction affects fibrillation and self-assembly properties, and in turn the final collagen biomaterial product.
2.2. Collagen Extraction
Collagen extraction depends on its solubility in the chosen solvent and composition of collagen types in the tissue sources [
26]. Collagen extraction can be broken down into 3 stages: Pre-treatment, extraction and purification. During the pre-treatment step, non-collagen proteins are removed to increase the yield of the collagen extraction process. Depending on the tissue source, removal of the non-collagen proteins (lipids, calcium, etc.) is achieved using alkali solutions, neutral saline solutions, alcohol solutions or a combination of solution [
33]. Following pre-treatment of tissues, collagen is then extracted via acid-solubilisation or enzymatic-digestion.
In the extraction of collagen by acid-solubilisation, the pre-treated tissue is added into a dilute acidic solution, typically acetic acid, to disrupt weaker hydrogen bonds between collagen molecules [
26]. This allows tissue swelling and acid-soluble collagen (ASC) from the loosened structure to dissolve in dilute acid [
34]. However, dilute acid does not disrupt the triple helix structure of collagen due to the strong intermolecular forces between the polypeptide strands [
35]. The extracted collagen still retains its telopeptide region and is known as telocollagen.
In the extraction of collagen by enzymatic-digestion, pre-treated tissue is added into a proteolytic enzyme solution, typically pepsin which cleaves non-helical telopeptide at the ends of the collagen microfibrils. Selective cleaving of the telopeptide region results in the destabilisation of the fibril structure and increases collagen dissolution [
34]. The triple helix structure of collagen is unaffected due to the selective pepsin enzyme digestion. The extracted collagen molecule does not retain its telopeptide regions and is known as atelocollagen.
While clinical use of collagen use both telocollagen as well as atelocollagen in dermal substitute product showed no collagen induced adverse immunogenic response, the removal the telopeptide regions is suspected to play a role in the immunogenicity and antigenicity of collagen [
36]. This is because the immune response in the body targets the antigenic determinant are found in mostly the telopeptides of collagen [
37]. However, the antigenic determinants which arise from the helical structure and the amino acid sequence of the collagen also contribute to the immunogenicity and antigenicity of collagen [
37]. Additionally, antigenic determinates for immune responses in the body depends on the species as well [
36].
These extraction methods are not exclusive and can be performed together. Enzymatic-digestion can be done on acid insoluble collagen to obtain higher yields [
26]. The extracted collagen is then filtered to remove impurities and purified through repeated salt precipitation, centrifugation and dissolution in acetic acid. Alternatively, the filtered extract undergoes dialysis for purification before freezing and freeze-drying.
2.3. Methods of Collagen Crosslinking
Additional crosslinking of collagen molecules can be used to enhance the mechanical properties of collagen to provide structural integrity for additive bio-manufacturing such as for muscle tissue (8–20 kPa), cartilage tissue (20–30 kPa) and bone tissue (2–30 GPa) [
45,
47]. These “artificial” crosslinking bonds can be generated using chemical agents or physical treatment. Increasing concentration of chemical and treatment times generally increase collagen crosslinking. However, when using chemical agents for crosslinker, residual unreacted chemicals and/or chemical byproducts are often left behind [
15]. This needs to be managed by washing to minimize cytotoxicity.
2.4. Collagen Analytical Methods
Understanding the structural, morphological, and chemical composition of collagen is critical since additive bio-manufacturing processes may give rise to significant changes. Understanding the structural, morphological and chemical composition allows better design and processing of the collagen raw material to meet the needs of the final product [
34,
77].
3. Collagen-Based Ink Printing Applications
3.1. Non-Additive Manufacturing
Non-additive manufacturing methods, casting and electrospinning of collagen-based inks and their applications are discussed. Casting involves pouring a liquid material into a mold of desired shape before solidifying and removal. Typically for collagen-based biomaterials, highly porous 3D structures (sponges) are obtained via the freeze-drying process while thin-films are obtained via air drying [
34]. Freeze drying is a complex process where ice crystals in the frozen mold are removed by sublimation under vacuum. Pore size and direction of the sponge can be controlled during freeze-drying [
96,
97].
Collagen sponges are used extensively in wound healing and tissue engineering as scaffolds for bone [
98], skin and soft tissues [
99]. The porous nature of collagen sponges allow cell migration as well as nutrient diffusion into the scaffold while providing a substrate for growth. The collagen sponge can be loaded with drugs, growth factors and bio-additives to enhance scaffold bioactivity [
50,
60,
98,
99,
100]. Collagen-glycosaminoglycan scaffolds have been successfully used to regenerate skin from full thickness burns [
50]. Additionally, by varying the glycosaminoglycan concentration and pore size, peripheral nerve tissue was successfully regenerated too [
101]. Loading TGF-β1 into a collagen sponge allowed controlled release of growth factors, enhancing bone regeneration of a rabbit skull defect [
44].
When collagen is laid out to dry, a thin-film of collagen is obtained via evaporation. As water and solvents evaporate, fibres and molecules are brought closer together due to surface tension of the solvent giving rise to a thin-film layer upon drying [
34]. Thin collagen films are typically used in cornea treatment owing to their optically transparent nature and biological properties [
61]. However, collagen films are not limited to ocular tissue engineering, micropatterns can also be designed onto the film as part of the casting process to influence osteoblast cell orientation [
54]. By stacking the collagen film layer by layer, the resulting biomatrix encouraged neo-tissue formation in a hernia repair model [
71]. The films can also be wrapped into tubes for nerve grafting applications [
49]. While functioning as a barrier membrane, collagen films can also be loaded with drugs, growth factors and bio-additives to enhance bioactivity. Additionally, collagen film degeneration and its mechanical properties can be controlled by varying crosslinking to control the release of its contents via degradation [
102,
103]. Collagen films are also suitable as edible food packaging [
104].
3.2. Additive Biomanufacturing
In this section, four additive bio-manufacturing technologies will be discussed: extrusion bioprinting, inkjet bioprinting, laser-assisted bioprinting and stereolithographic/digital light processing bioprinting. The main advantage of additive bio-manufacturing is to produce complex shapes with internal structures at high resolution and accuracy without molds or shaping tools required by non-additive methods. Moreover, additive bio-manufacturing is amenable to printing with cell-laden inks (bio-inks) [
24].
While all additive biomanufacturing processes create structures via layer-by-layer deposition of biomaterials, not all collagen-based inks can be printed using the following methods. As such, flexible printing method such as extrusion printing have a larger number of applications and variation of printing formulations, whereas more restrictive printing methods such as inkjet, laser-assisted, and stereolithography printing have fewer applications.
To aid the reader, Table 1 has been provided to summarise applications of additive bioprinting methods for collagen biomaterials/biocomposites and bioinks (cell-laden).
Table 1. Applications of additive bioprinting methods for collagen-based inks.
|
Bioprinting Method
|
Collagen-Based Ink Formulation
|
Outcome
|
Ref.
|
|
Extrusion
|
Methacrylated type I collagen; Sodium alginate
|
Fabrication of structures that resembles native human corneal stroma with cell-laden bioink via extrusion bioprinting.
|
[116]
|
|
Extrusion
|
Collagen Type I; Alginic acid sodium salt from brown algae; CaCl2 solution
|
Core-sheath coaxial extrusion of alginate/collagen bioink with CaCl2 allows creation of scaffolds with low collagen centration despite its low viscosity.
|
[114]
|
|
Extrusion
|
Rat tail type I collagen; Gelatin (type A); Sodium alginate
|
Extrusion bioprinting of collagen scaffold via gelatin/alginate system with controllable degradation time based on amount of sodium citrate during incubation.
|
[113]
|
|
Extrusion
|
Type I collagen was extracted from tendons obtained from rat tails
|
Identified storage modulus as the best predictor of collagen bioink printability during deposition.
|
[117]
|
|
Extrusion
|
PureCol Purified Bovine Collagen Solution; Soldium alginate (low viscosity)
|
Fabrication of interwoven hard (PLLA) and soft (bioink) scaffolds which support cell attachment and proliferation using a modified desktop 3D printer.
|
[135]
|
|
Extrusion
|
Methacrylated COL I; Heprasil; Photoinitiator
|
Successful bioprinting of liver model. Printed primary hepatocytes retained function over 2 weeks exhibiting appropriate response to toxic drugs.
|
[41]
|
|
Extrusion
|
Lyophilized Atelo-collagen, Matrixen-PSP
|
Pre-set extrusion bioprinting technique is able to create heterogeneous, multicellular and multi-material structures which perform better than traditional bioprinting.
|
[112]
|
|
Extrusion
|
Collagen Type I extracted from rat tails; Pluronic® F127
|
Fabrication of 3D constructs without chemical or photocrosslinking before and after printing via thermally-controlled extrusion.
|
[115]
|
|
Extrusion
|
Lyophilized sterile collagen, Viscoll
|
Formation of scaffolds which support spatial arrangement of tissue spheroids as well as support cell adhesion and proliferation.
|
[47]
|
|
Extrusion
|
Type-I collagen, Matrixen-PSP; Tannic acid
|
Fabrication of 3D porous structures which support cell migration and proliferation for long periods of culture. Determined optimal tannic acid crosslinking.
|
[67]
|
|
Extrusion
|
Collagen Type I; Sodium Alginate
|
Improved mechanical strength and bioactivity via the addition of collagen. Higher cartilage gene markers expressed, preservation of chondrocyte phenotype.
|
[42]
|
|
Extrusion
|
Type-1 collagen, Matrixen-PSP
|
Established a crosslinking process using tannic acid. High printed preosteoblast viability and well-defined pore size and strut dimensions for bone regeneration.
|
[68]
|
|
Extrusion
|
Type-I collagen, Matrixen-PSP; Decellularised extracellular matrix (dECM); Silk Fibroin(SF)
|
Hybrid collagen/dECM/SF scaffold with enhanced cellular activity and mechanical properties. Enhanced cell differentiation, mechanical properties, amenable for hard tissue regeneration.
|
[59]
|
|
Extrusion
|
Atelocollagen Type I powder
|
Novel self-assembly induced 3D printing to produce macro/nano porous collagen scaffolds with reasonable mechanical properties, excellent biocompatibility and mimicking native ECM.
|
[58]
|
|
Extrusion
|
Type-I collagen, Matrixen-PSP; Polycaprolactone (PCL); Hydroxyapatite (HA)/β-tricalcium-phosphate (TCP); Platelet-rich plasma(PRP)
|
Fabrication of collagen/PCL biocomposites loaded with bio-additives via 3D extrusion printing. Collagen/PCL biocomposites allow controlled release of HA/TCP bio-additives, which promote osteogenesis. PRP biocomposites demonstrate increased mineralisation.
|
[46]
|
|
Extrusion
|
Type-I collagen, Matrixen-PSP
|
Genipin crosslinking allowed fabrication of 3D cell-laden porous scaffold (Cellblock) with mechanical stability, pore size and osteogenic (bone tissue regeneration) potential.
|
[70]
|
|
Extrusion/Inkjet
|
Lyophilized collagen type 1 sponge derived from porcine skin
|
Development of a one-step process to produce a 3D human skin model with functional transwell system. Cost-effective compared to traditional transwell cultures.
|
[118]
|
|
Inkjet
|
Type I rat tail collagen; poly-d-lysine
|
Fabrication of neuron-adhesive patterns by printing cell-adhesive layers onto cell-repulsive substrates.
|
[123]
|
|
Inkjet
|
Collagen (Calf skin)
|
Cell aggregates printed between layers of collagen gels suitable for tissue engineering.
|
[125]
|
|
Inkjet
|
Collagen (rat-tail); collagen (calf skin)
|
Low-cost, high-throughput surface patterning with collagen and potentially, other proteins.
|
[122]
|
|
Inkjet
|
Collagen Type I
|
Fabrication of in vitro cancer microtissues via collagen inkjet printing. Four individual microtissues within one 96-well plate well, maintained for up to seven days.
|
[124]
|
|
Inkjet
|
Collagen: Type I rat tail collagen; Fibrinogen; Thrombin
|
Collagen bioinks and Fibrin/Collagen bioinks unsuitable for in situ inkjet bioprinting.
|
[136]
|
|
Inkjet
|
Type I acidic collagen; Agarose (low gelling temperature)
|
Fabrication of 3D corneal stromal structure with optically properties similar to native corneal stroma. Potential as a clinical or experimental model.
|
[120]
|
|
Inkjet
|
Acidic collagen solution; Agarose (low gelling temperature)
|
MSC branching, spreading and osteogenic differentiation controlled by collagen concentration; Osteogenic potential (bone tissue engineering).
|
[121]
|
|
Laser-assisted
|
Collagen Type I (Rat-tail)
|
Fabrication of cell-laden skin tissue using laser-assisted bioprinting, in vivo potential. Skin tissues consist of: a base matriderm layer, 20 layers of fibroblast and 20 layers of keratinocytes.
|
[130]
|
|
Laser-assisted
|
Collagen (Rat-tail)
|
Multicellular collagen skin tissue constructs printed using laser-assisted bioprinting. Keratinocyte and fibroblast layers did not intermix after 10 days. Mimics tissue-specific functions (e.g., gap-junction).
|
[129]
|
|
Laser-assisted
|
Type I collagen (rat) solution; Nano hydroxyapatite (nHA)
|
In situ printing of cell-laden collagen-based ink via laser assisted bioprinting allow bone regeneration (mouse calvaria defect model). Contact free printing method is sterile with clinical potential.
|
[126]
|
|
Laser-assisted
|
OptiCol™ human Col I; Ethylenediaminetetraacetic acid (EDTA) human female AB blood plasma; Thrombin from human plasma
|
Fabrication of 3D cornea tissue using novel human protein bioinks via laser assisted bioprinting. Novel bioink is biocompatible, without requiring additional crosslinking. First study to demonstrate laser-assisted bioprinting for corneal applications using human stem cells.
|
[131]
|
|
Stereolithography (SLA)
|
Collagen methacrylamide(CMA) synthesized using Type-I collagen; Irgacure (I2959)
|
Free-form photolithographic fabrication; photopatterned hydrogels retain structure after 24 h. CMA retains native collagen self-assembling properties; hydrogels biocompatible in vivo.
|
[134]
|
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering7030066