Plants are exposed to highly fluctuating effects of light, temperature, weather conditions, and many other environmental factors throughout their life. As sessile organisms, unlike animals, they are unable to escape, hide, or even change their position. Therefore, the growth and development of plants are largely determined by interaction with the external environment. The success of this interaction depends on the ability of the phenotype plasticity, which is largely determined by epigenetic regulation. In addition to how environmental factors can change the patterns of genes expression, epigenetic regulation determines how genetic expression changes during the differentiation of one cell type into another and how patterns of gene expression are passed from one cell to its descendants. Thus, one genome can generate many ‘epigenomes’. Epigenetic modifications acquire special significance during the formation of gametes and plant reproduction when epigenetic marks are eliminated during meiosis and early embryogenesis and later reappear. However, during asexual plant reproduction, when meiosis is absent or suspended, epigenetic modifications that have arisen in the parental sporophyte can be transmitted to the next clonal generation practically unchanged. In plants that reproduce sexually and asexually, epigenetic variability has different adaptive significance.
1. Epigenetic Systems in Plants
In the last two decades, much data on the epigenetic regulation of plants have appeared, as well as works summarizing the accumulated knowledge
[1][2][3][4]; nevertheless, many questions remain unclear, and a number of results are contradictory. New in this area, data are constantly emerging. We tried to take into account and discuss the main findings and conclusions in this field.
Epigenetics, ‘epi’ (upon, above, beyond) and ‘genetic’ (DNA sequence), refers to a layer of information that exists beyond that encoded in the DNA sequence. Therefore, although, in a given organism, the complement of DNA is essentially the same in all somatic cells, patterns of gene expression differ greatly among different cell types, and these patterns can be clonally inherited. Today the term epigenetics also include transient mechanisms separate from the one required to maintain it. Epigenetic regulation of gene expression is understood primarily as DNA methylation, modification of histones by methylation, acetylation, and ubiquitination of histone N-tails, and post-transcriptional silencing through small non-coding RNAs. In the model plant Arabidopsis, more than 130 genes are known that are regulated epigenetically
[2].
In plants, 5-cytosine is the main site of DNA methylation. The latter occurs in the three contexts, symmetric CH, CHG, and asymmetric CHH, where H is any nucleotide except G. In the symmetrical context, the most frequently methylated are repetitive motives of CG or CHG, referred to as CG islands. CG methylation during replication cycles is supported by the enzyme methyltransferase 1 (MET1) in a semi-conserved way. Methylation in the context of CHG is supported by chromomethylase 3 (CMT3) methyltransferase. Due to its asymmetric nature, CHH must be methylated de novo after each round of replication. CHH methylation sites are catalyzed by domain-rearranged methyltransferase 2 (DRM2), which is involved in plant-specific RNA-directed DNA methylation (RdDM). CHH methylation can also be performed by chromomethylase 2 (CMT2) homologous to CMT3 independently of RdDM. The enzyme decrease in DNA methylation 1 (DDM1) remodels chromatin by removing the histone H1 linker in the compact heterochromatic regions, providing access to methyltransferases to DNA. DRM2-mediated methylation is mainly involved in the methylation of euchromatic regions, including short transposable elements (TE) and edge fragments of long TE, as well as pericentromeric sites
[5].
An important role in the biogenesis of small interfering RNAs is played by polymerase IV and polymerase V, which are plant homologs of polymerase II, and that specialize in the production of small RNAs required for RdDM. Polymerase IV synthesizes single-stranded RNA (ssRNA) on silencing targets, retrotransposons, viruses, transgenes, or repetitive genes. RNA-dependent RNA polymerase 2 (RDR2) promotes the formation of double-stranded RNA (dsRNA) from ssRNA. Next, diser-like 3 (DCL3) cuts dsRNA into 24- and 23-nt small interfering RNAs (siRNAs), one strand of the duplex are loaded to Argonaute (AGO4). AGO4-bound siRNAs complement with polymerase V transcripts and recruit DRM2, which catalyzes de novo methylation of the genome homologous sites in all contexts
[6][7].
Active DNA demethylation occurs via DNA glycosylase repressor of silencing 1 (ROS1), demeter (DME), demeter-like 2 (DML2), and DML3. The mode of action of glycosylases is through the elimination of methylated cytosine, replacing it with non-methylated cytosine. During plant reproduction and further ontogenesis, various modes of dynamic DNA methylation and demethylation in all contexts provide the necessary genetic regulation of development
[8].
In addition to DNA methylation, the modification of histones, nuclear proteins involved in the packaging of DNA strands in the nucleus and in the epigenetic regulation of transcription and replication is of great importance for genetic regulation. Chromatin remodeling (a change in its structure) occurs, among other things, due to histone modifications. It alters the availability of DNA for transcription factors and polymerases, thereby regulating gene expression and contributing to phenotype variability. Like DNA methylation, chromatin remodeling plays an important role in plant reproduction and ontogenesis. Basically, the epigenetic marks are the methylation of lysines in histone H3. Thus, the trimethylation of lysine 3 and lysine 4 in histone H3 (H3K4me3 and H3K3me3, respectively) leads to the formation of “active” chromatin, which allows genes to be expressed, while dimethylation of lysine 9 and trimethylation of lysine 27 (H3K9me2 and H3K27me3, respectively) produces repressive chromatin, which suppresses the transcriptional activity of genes. The formation of repressive chromatin containing H3K27me3 regulates the evolutionarily conserved complex of proteins polycomb repressive complex 2 (PRC2), which regulates many developmental processes of reproduction and the initial stages of seed formation in plants.
Histone acetylation is usually an epigenetic mark associated with active chromatin and transcriptional activity
[9].
2. Methylation in the Meristem Development
A distinctive feature of plant morphogenesis is the totipotency of some cells of plant meristems, which perform the function of stem cells. These cells can transform into any cell of plant tissue or organ. Since meristems persist throughout the whole life of a plant, new organs and tissues can form indefinitely, and in this sense, plants are immortal. In other words, clones of plant genotypes can exist indefinitely through vegetative propagation, as well as by in vitro propagation in tissue culture. In plants, in addition to the shoot apical meristem, which gives rise to leaves, stem, and flower buds, there are also lateral, intercalary, and marginal meristems, whose cells, under certain conditions, are capable of organogenesis and somatic embryogenesis. In Waddington’s terms, meristematic totipotent plant cells are polyvalent and can follow many trajectories of the epigenetic landscape
[10][11].
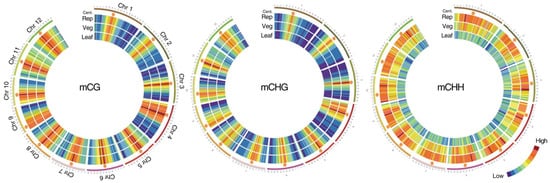
During the transition from the vegetative to the generative phase of development in the shoot apical meristem (SAM), the patterns of DNA and histone methylation change significantly (
Figure 1)
[12]. After epigenetic modifications occur in the stem cells of the meristems due to gradients of phytohormones and interaction with the environment, the shoot apical meristem turns into a flower meristem, in which ovary formation and meiosis take place. Unlike animals, plants do not form germ lines directly; instead, they form haploid male and female gametophytes in which gametogenesis occurs. Since male and female gametophytes are haploid, possible harmful mutations that have arisen are not compensated by the homologous allele, and gametophytes carrying such mutations are aborted
[13]. However, zygotic embryoletal and sporophytic mutations can pass through the gametophyte stage since their function becomes noticeable only at the zygote/embryo and sporophyte (diploid plant) stages, respectively. These mutations can be transmitted heterozygous in segregated populations
[13]. Due to the peculiar gametogenesis and embryogenesis in plants, many epigenetic marks associated with the suppression of transposons are temporarily suppressed by small non-coding RNAs of neighboring tissues
[14][15].
Figure 1. Methylation in the vegetative and reproductive SAMs. Heat maps showing cytosine methylation levels in the 12 rice for the CG (
left), CHG (
middle), and CHH (
right) contexts. SAM (Veg)—vegetative meristem, SAM (Rep)—reproductive meristem, (Leaf)—leaf. Orange hexagons mark pericentromeric regions. Modified from the work of
[12].
Perhaps the first epiallele in nature was found in toadflax (
Linaria vulgaris) when Karl Linnaeus studied the shape of the flower. In wild-type plants, the flower shape is zygomorphic, that is, bilaterally symmetric, while several peloric flowers develop radial symmetry, i.e., become actinomorphic (
Figure 2). Genetic analysis showed that radial symmetry arises because of methylation of the
Lcyc locus, while transcripts of this gene are not found in actinomorphic flowers
[16].
Lcyc is homologous to the
Cycloidea gene, which controls the symmetry in the snapdragon flower
[17].
Figure 2. Actinomorphic (peloric) and zygomorphic (most typical) flowers in toadflax (Linaria vulgaris). The symmetry and shape of the flower are determined by the methylation of the Lcyc gene.
Another well-known example of epiallele is the
CNR (Colorless non-ripening) locus in tomatoes
[18]. In the mutants with this epiallele, seed maturation is suppressed, and colorless mealy fruits are formed. Genetic analysis showed that in individuals containing the mutant allele, the SBP transcription factor is poorly expressed due to the high methylation of the upstream region of the
Le SPL-CNR gene.
Epimutations occur much more often and faster than proper mutations. However, unlike the latter, epimutations are reversible and thus are of great importance in the quick tuning and plasticity of the phenotype. Epigenetic diversity can provide phenotypic variability, which is valuable both for the fitness of the plant populations to changing environments and could be used for breeding agricultural plants.
3. Epigenetic Regulation of Microsporogenesis and Male Gametophyte Development
As already mentioned, in plants, primary germ cells do not directly enter spermatogenesis and oogenesis, in contrast to animals. Instead, in the flower meristem, pollen mother cells (PMCs) in anthers and megaspore mother cells (MMCs) in ovaries are formed as a result of two meiotic divisions followed by the series of mitotic divisions producing haploid male and female gametophytes, where male and female gametes origin, respectively
[17][18]. In angiosperms, male and female gametophytes, despite their small size and small number of cells, are excellent models for studying morphogenesis and epigenetic control of cell growth and specialization, cell polarity, and signaling processes.
In the case of pollen development, which occurs in the anthers, two stages can be distinguished, microsporogenesis and microgametogenesis. Diploid microsporocytes, or PMCs, are formed in the sporogenous layer of the anther. Two meiotic divisions of PMC produce tetrad of four haploid cells. Then tetrads separate into individual microspores. Afterward, two mitotic divisions take place: in the first asymmetric cytokinesis forms a large vegetative and a smaller generative cell, followed by the generative cell division producing two sperm cells, while the vegetative cell does not divide anymore. So, mature pollen grain consists of three cells, a larger vegetative cell, which controls and implements the growth of the pollen tube during fertilization, and two smaller sperm cells, which take part in double fertilization
[19].
Epigenetic rearrangements play an important role in the regulation of both male and female gametophytes development, as well as in fertilization
[14][20][21][22][23].
In Arabidopsis, it has been shown that in the PMC methylation level in a symmetric context, CG and CHG, was higher compared to asymmetric context, CHH
[20]. It is known that symmetric methylation occurs mainly in transposable elements, while asymmetric hypermethylation usually takes place in protein-coding genes. It is likely that increased methylation in a symmetric context promotes suppression of TE activity, which ensures genome stability before and during meiosis. At the same time, inactivation of methylation in an asymmetric context promotes the activation of genes necessary for launching sperm cell formation programs and further fertilization. In addition to DNA methylation, during the maturation of PMCs, a dramatic reorganization of chromatin occurs, which promotes the commence of meiosis. The change from the mitotic to the meiotic phase is accompanied by an increase in permissive chromatin (H3K4me3) and a reduction in repressive chromatin (H3K27me1 and H3K27me3)
[24]. After meiosis and asymmetric mitotic division of the haploid microspore, the vegetative cell becomes roundish. It has an increased level of methylation in the CHH regions, while it significantly loses the centromere-specific histone H3 (CENH3) because of the decondensation of pericentromeric heterochromatin, local hypomethylation due to the activity of DME/ROS1 demethylases, and activation of transposable elements is also observed
[14][15][25]. The centromeric histone H3 (CENH3) variant plays an important role in the assembly and function of kinetochores during mitotic and meiotic cell division. Kinetochore assembly begins with the incorporation of CENH3 into centromeric nucleosomes. The deposition of CENH3 on centromeres varies with the stage of the cell cycle. CENH3 is also essential for vegetative cell division and further DNA elimination
[26]. Hypomethylation of TEs results in the generation of 21–22 nt siRNAs, which are transported to the sperm cells to suppress their TEs by RdDM methylation
[14]. In general, whole-genome cell-specific methylation profiling revealed a high level of CG and CHG methylation in the DNA of microspores, sperm, and vegetative cells during the entire period of pollen formation and development. While most of the CHH methylation is lost in the pericentromeric region of microspores and sperm cells, it is restored in the vegetative cells.
4. Epigenetic Regulation of Megasporogenesis and Female Gametophyte Development
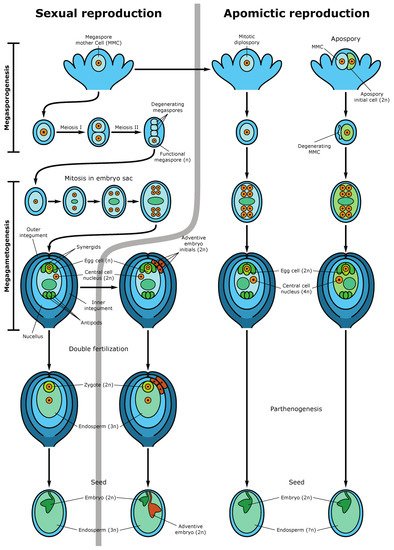
The formation of an angiosperm egg cell begins with the development of archesporial cells from the subepidermal layer of the flower bud’s nucellus. Archesporial cells produce a diploid dense cytoplasmic, large nuclear megaspore mother cell (MMC), or megasporocytes. MMC undergoes two meiotic divisions, forming a tetrad of megaspores, one of which is referred to as functional megaspores, passing through three rounds of mitosis, produces an eight-nuclear megagametophyte, also called an embryo sac. After cellularization, the embryo sac becomes seven cellular: at one pole, there is an egg apparatus, consisting of an egg cell and two synergids, the latter contribute to the attraction of the sperm cell and fertilization, and at the other pole, there are three antipodal cells that take part in the nutrition of the embryo sac
[13][27]. In the center of the embryo sac, there are two polar nuclei, which as a result of karyogamy, fuse to form the homodiploid nucleus of the central cell. During fertilization, the pollen tube enters the embryo sac in the egg apparatus area, and one sperm fertilizes the egg cell, forming a diploid zygote, from which the embryo (new sporophyte) then develops, and the second sperm fuses with the central cell nucleus, producing a triploid nucleus of the first endosperm cell (nourishing tissue for the embryo) (
Figure 3). This process is called double fertilization.
Figure 3. Sexual and apomictic seed development. The left side demonstrates the sexual pathway and sporophytic apomixis (adventive embryony). In the sexual pathway, the megaspore mother cell (MMC) undergoes two meiotic divisions producing a tetrad of haploid megaspores. Three megaspores degenerate while the functional megaspore gives rise to the embryo sac (female gametophyte) following three rounds of mitotic divisions. The mature embryo sac consists of 7-cells (8 haploid nuclei) in which the egg cell and two synergid cells are located at one pole of the embryo sac and three antipodal cells at the opposite pole. Two polar nuclei fuse to form the diploid nucleus of the central cell. During double fertilization, one sperm fertilizes the egg cell, forming the diploid zygote and the second sperm fertilizes the central cell producing the first nuclei of the triploid endosperm. Synergids participate in the perception of the sperm cells and burst after fertilization. The mature seed consists of the diploid embryo, the triploid endosperm (nourishing tissue), and the seed coat. An adventive embryo may develop from the nucellar or inner integumental sporophytic tissues and develops alongside the sexual embryo. The right part of the figure shows gametophytic apomixis. Meiosis and chromosome reduction are missing. Embryo sac development is initiated from an unreduced MMC, which gives rise to not haploid tetrads but diploid cells of the dyad (diplospory), or from an apospory initial cell (apospory). The embryo develops parthenogenetically from the unreduced egg cell while the endosperm is formed either autonomously or through fertilization of the central cell (in the case of pseudogamy). The mature apomictic seed contains the diploid embryo, and the endosperm ploidy may vary but usually not less than 4n
[27].
During megasporocyte formation (MMC), the level of DNA methylation temporarily decreases in the context of CHH, but methylation in the CG context remains basically unchanged
[21]. The specification and differentiation of the MMC, as well as the functional megaspore, is carried out, inter alia, through intercellular interactions by the mobile trans activating siRNAs (tasiRNAs) produced in the surrounding cells of nucellus and transported to the MMC, where they implement silencing at the transcriptional and translational level
[28]. It was shown in Arabidopsis that the formation of such siRNAs is regulated by AGO9, RDR6, and SDS3 (a suppressor of genetic silencing 3) enzymes
[29]. Disruption of AGO5 expression in Arabidopsis nucellus impairs the initiation of megagametogenesis.
Methylation in the CG and CHH contexts remains stable throughout megagametogenesis. At the same time, as was found in Arabidopsis, CG methylation within genes and transposons of the central cell of the embryo sac was lower than that in sperm cells
[30]. Demethylation of DNA in the central cell was performed by the demeter activity
[31]. This may indicate that the potential transcription of male genes is suppressed even before fertilization. During gametogenesis in the embryo sac, epigenetic regulation takes place by mobile non-coding tasiRNAs, so siRNAs from the central cell enter the egg cell and suppress the activity of transposable elements. The foregoing confirms the importance of epigenetic control in megasporgenesis and megagametogenesis.
This entry is adapted from the peer-reviewed paper 10.3390/epigenomes5040025