Despite advances in antimicrobial therapy and even the advent of some effective vaccines, Pseudomonas aeruginosa (P. aeruginosa) remains a significant cause of infectious disease, primarily due to antibiotic resistance. Although P. aeruginosa is commonly treatable with readily available therapeutics, these therapies are not always efficacious, particularly for certain classes of patients (e.g., cystic fibrosis (CF)) and for drug-resistant strains. Combinations of monoclonal antibodies against different targets and epitopes have demonstrated synergistic efficacy with each other as well as in combination with antimicrobial agents typically used to treat these infections. Such a strategy has reduced the ability of infectious agents to develop resistance. This review highlights potential targets secreted by P. aeruginosa that future polyclonal antibodies may directed against in order to develop more efficacious treatments against these infections.
1. Introduction
P. aeruginosa is a Gram-negative bacillus implicated in a wide variety of human infections. In acute infections, individual
P. aeruginosa organisms exhibit swarming motility via a single flagellum and type 4 pili and express a wide variety of toxins, cell surface proteins, and other molecules that contribute to its immunogenicity and pathogenicity [
1]. In order to establish chronic infection,
P. aeruginosa transitions to a sessile, non-motile state marked by the formation of a mucoid biofilm, composed mainly of exo-polysaccharides, glycolipids, and mucin, which often poses a barrier to successful clinical treatment [
2]. Regardless of if
P. aeruginosa exists in an acute motile form or a chronic sessile biofilm, infection with
P. aeruginosa is particularly perilous for immunosuppressed patients [
1], ventilator-dependent patients, and cystic fibrosis patients.
Additionally,
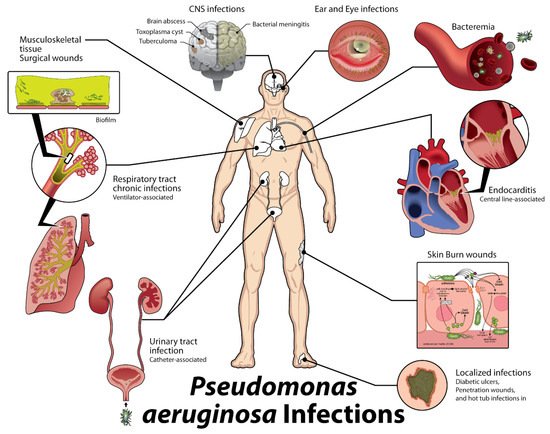
P. aeruginosa has been recognized as the causative organism in catheter-associated urinary tract infections, otitis externa, otitis media, contact lens keratitis, soft tissue infections in burn victims and AIDS patients, septic arthritis, folliculitis, meningitis, and sepsis. In fact, this broad array of associated disease states (
Figure 1) has led
P. aeruginosa to be recognized as the sixth leading cause of hospital-acquired infections, the second most common cause of ventilator-associated pneumonia and the most common multidrug-resistant Gram-negative cause of ventilator-associated pneumonia, the third most common cause of catheter-associated UTI, and the fifth most common cause of surgical site infections [
1].
Figure 1. Types of Acute
P. Aeruginosa Infections [
5].
P. aeruginosa is prevalent in skin and soft tissue infections (top right) including trauma, burns, and dermatitis. It also commonly causes swimmer’s’ ear (external otitis), hot tub folliculitis, and ocular infections, bacteremia and septicemia, especially in immunocompromised patients, and endocarditis associated with IV drug users and prosthetic heart valves (bottom right).
P. aeruginosa can also cause central nervous system (CNS) infections such as meningitis and brain abscess (top left), bone and joint infections, including osteomyelitis and osteochondritis, respiratory tract infections, and hospital-acquired urinary tract infections (UTIs; bottom left).
P. aeruginosa is also resistant to many common antibiotics [
5].
The vast array of infectious complications that can arise from normal commensal and environmental strains of
P. aeruginosa indicates that it is an opportunistic, adaptable, common environmental pathogen, making
P. aeruginosa very robust and difficult to treat. Several antimicrobial agents possess the ability to treat
P. aeruginosa infections [
3]; however, successful clinical treatment regimens should include pre-treatment sensitivity testing, as different strains possess widely different antimicrobial resistances. Importantly, treatment is often dictated by the antibiogram of a specific hospital or region.
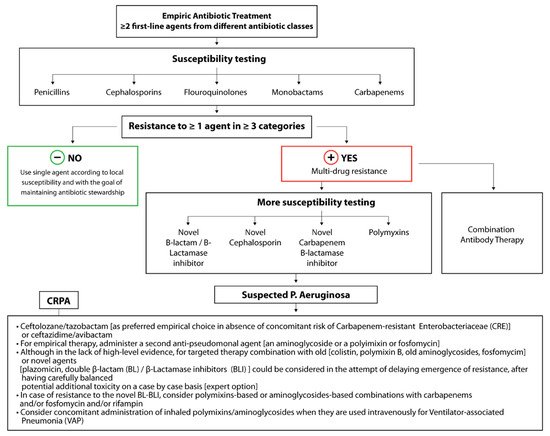
P. aeruginosa is often susceptible to first-line agents, including beta-lactam antibiotics (e.g., piperacillin-tazobactam and ticarcillin-clavulanate), cephalosporins (e.g., ceftazidime, cefoperazone, and cefepime), and monobactams (e.g., Aztreonam). Carbapenems (e.g., meropenem and doripenem), which were historically seen as the “big gun”, last-ditch antimicrobials, can be used to treat highly resistant infections. However, as of 2019, the World Health Organization has listed carbapenem-resistant
P. aeruginosa as one of three bacterial diseases in critical need of new treatment strategies, with up to 14% of
P. aeruginosa isolates in the U.S. in 2019 expressing carbapenem resistance (
Figure 2) [
6].
Figure 2. Treatment strategy for carbapenem-resistant
P. aeruginosa isolates including future treatment options based on combinatorial antibody therapies [
21].
In the ongoing battle between humans and the pathogenic microbes that cause disease, the CDC recognizes that the development of newer antimicrobial pharmacotherapeutics continues to be a pressing need, despite several current pharmaceutical agents that are reserved for the treatment of multidrug-resistant isolates [
7]. In response to advancing antimicrobial pharmacotherapies, particularly bactericidal therapies that impose selective pressure, bacterial resistance mechanisms continue to evolve as opportunistic microbes adapt to an ever-changing therapeutic landscape. The evolution of multidrug-resistant
P. aeruginosa can be considered as a case study based on its sophisticated
quorum sensing communication system and phenotypic plasticity that has allowed it to adapt, survive, and thrive in a wide variety of environmental (e.g., aquatic and soil) and host conditions [
11]. Among clinical isolates, a wide range of phenotypic variation has been identified including hyperpigmentation, small colony variant formation, autoaggregation, alginate overproduction, and autolysis [
12,
13,
14,
15,
16]. These phenotypes change and adapt as an infection progresses, allowing for long-term survival in the differing conditions of the host [
17].
2. Host Immune Response
P. aeruginosa infection commonly induces a robust humoral response including IgG antibodies towards lipopolysaccharide (LPS), alginate, alkaline protease, elastase, exotoxin A, and many other surface antigens and proteins of
P. aeruginosa, which are often upregulated as virulence factors during various stages of biofilm development [
36,
37]. Unfortunately, the host antibodies produced typically have low affinity for their respective targets and are not effective at eliminating the infection [
25]. As an aside, host opsonizing antibodies also cannot eliminate these mucoid microorganisms [
38]. Nevertheless, anti-
P. aeruginosa IgG binds to its antigen and immune complexes are formed, activating complement and recruiting macrophages. As macrophages and immune cells bind the anti-
P. aeruginosa IgG, they create reactive oxygen species (ROS), consuming oxygen, making the biofilm environment more anaerobic and thus more favorable for the organism. The anaerobic environment is unsuitable for host macrophages, neutrophils, and other immune cells. Phagocytosis may occur, but without sufficient oxygen, ROS cannot be produced to eliminate bacteria. This creates an inflammatory environment causing tissue damage without efficient disruption of
P. aeruginosa biofilms [
25]. The ongoing inflammatory state in chronic infections is thus not linked to immunogenicity of the bacterial organisms themselves, but rather the secreted products that leave the biofilm and induce immune responses in the airway epithelium [
23,
24]; hence, the humoral responses produced by many people in response to
P. aeruginosa infection are not effective in eliminating the infection.
3. Description of Targets
Taking inspiration from the immune response and in the context of P. aeruginosa’s life cycle and its antibiotic resistance mechanisms, several potential targets secreted by P. aeruginosa were identified. These targets, outlined in Table 1, produce a wide variety of effects in hosts and the bacteria, contributing to the pathogenesis of the entire spectrum of infections caused by this organism.
Table 1. Potential Therapeutic Antibody Targets.
| Location or Class |
Examples |
Activity/Effects on Host |
| Cell surface |
Alginate |
Antiphagocytic, resists opsonic killing |
| Lipopolysaccharide |
Endotoxic, antiphagocytic, avoids preformed antibody to previously encountered O antigens |
| Pili (produced by type IV secretion) |
Twitching motility, biofilm formation, adherence to host tissues |
| Flagella |
Motility, biofilm formation, adherence to host tissues and mucin components |
| Injection of type III secretion factors |
PcrG, PcrV, PcrH, PopB, and PopD proteins form injection bridge for type III effectors |
| Outer membrane |
Siderophore receptors |
Provides iron for microbial growth and survival |
| |
Efflux pumps |
Remove antibiotics |
| Secretion systems |
|
|
|
|
Elastase, lipase, phospholipases, chitin-binding protein, exotoxin A, and others |
Variety of proteolytic, lipolytic, and toxic factors; degrade host immune effectors |
|
|
ExoS, ExoT, ExoU, ExoY |
Intoxicates cells (ExoS, ExoT); cytotoxic (ExoU); disrupts actin cytoskeleton |
|
|
Cytoplasmic and membrane-associated proteins, ATPases, lipoproteins, Hcp1 protein |
Poorly characterized but found in animal studies to be needed for optimal virulence, particularly in chronic infection |
| Iron acquisition |
Pyoverdin, pyochelin, HasAP |
Scavenge iron from the host for bacterial use |
| Secreted toxins |
Hemolysins, rhamnolipid phospholipases |
Kill leukocytes, hemolysis of red cells, degrade host cell surface glycolipids |
| Secreted oxidative factors |
Pyocyanin, ferric pyochelin, HCN |
Produce reactive oxygen species: H2O2, O2−
Inflammatory, disrupts epithelial cell function |
| Quorum sensing |
LasR/LasI, RhlR/RhlI, PQS |
Biofilm formation, regulation of virulence factor secretion |
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics10121530