Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Allergy
Ovarian cancer (OCa) is characterized as one of the common reasons for cancer-associated death in women globally. This gynecological disorder is chiefly named the “silent killer” due to lacking an association between disease manifestations in the early stages and OCa.
- curcumin
- herbal medicine
1. Introduction
Ovarian cancer (OCa) is characterized as the fifth most prevalent reason for death in women around the world because of its insidious initiation, weak prognosis, and rapid development. Based on estimations, annually, more than 100,000 females die due to OCa globally [1]. Mainly, OCa is categorized into three types: germ cell, sex-cord-stromal, and epithelia [2]. The most common form of OCa is epithelial OC (EOC), which is a heterogenic disorder, and histologically, EOC can be divided into four main subgroups, endometrioid, serous, mucinous, and clear cell carcinomas [3]. The etiology of OCa has not been completely illustrated yet, but it is shown that obesity, hereditary, aging, alcohol consumption, smoking, and diabetes mellitus are risk factors of OCa [4,5]. Plus, it is expressed that environmental, hormonal, and ovulation factors may have a role in the pathogenesis of OCa [6]. According to reports, OCa progression is linked with various pathways that interfered with the metabolism of energy, like galactose metabolism, which is related to the risk of OCa development [7]. OCa is mostly named the “silent killer” since the observed manifestations in the early stages of the disease are not clearly related to OCa [8]. The signs and symptoms of OCa can be general and ambiguous, such as abdominal pain, abnormal bowel habits, and early satiety [9]. There is a substantial need for using novel therapies for OCa due to disease recurrence and resistance to common therapies, such as chemotherapy and surgery [6,10,11]. In addition, these approaches can significantly along to cytotoxic impacts and severe complications [8]. Therefore, finding and using efficient curative methods against this gynecological tumor is indispensable. Among these, herbal remedies have obtained great attention from thousand years ago owing to their effectiveness against different ailments, such as cancer [12]. It is stated that some herbal compounds, like phenols, alkaloids, and lectins, can exert anti-cancer effects by apoptosis induction [13]. On the other hand, nano-based drug delivery systems, such as nanoparticles (NPs), nano micelles, liposomes, branched dendrimers, nanostructured lipid formulations, and nanocapsules, have been developed recently for the treatment of OCa [14]. Thus, the combination of herbal therapy and nano-based therapy may provide a new horizon in the improvement of OCa.
2. Ovarian Cancer and Its Pathogenesis
Presently, the pathogenesis and clinicopathological properties of OCa have not clearly been expressed [18]. However, some theories have been proposed concerning OCa origination (Figure 1) which includes (1) the gonadotropin theory characterizes over the induction of the epithelium of ovarian surface by hormonal receptors resulting in malignancy, (2) the continuous ovulation theory, in the location of which the cells of ovarian surface epithelium are damaged because of constant ovulation, (3) the origin cells for the majority of epithelial ovarian cancers are not derived from the ovary but mostly originated from the fallopian tube and develop to the ovary and more than it [19]. Regarding hormonal conditions, there is evidence that progesterone and androgens can elevate ovarian epithelium proliferation and subsequently lead to the formation of OCa. Indeed, the increment of androgens and estrogens taggers multiple pro-inflammatory agents resulting in immune activation. During ovulatory occurrences, a great number of molecules are produced, such as chemokines and cytokines, plasminogen activators, prostaglandins, interleukins, bioactive eicosanoids, tumor necrosis factors, collagenases, and a large number of growth factors and immune cells, which all trigger a pro-inflammatory occurrence. Such pro-inflammatory agents, like IL-8, CCL2/MCP-1, and CCL5/RANTES, are induced during each cycle of ovulation; therefore, the continuous ovulation theory recommends that the inflammation accompanied by other physiological situations potentiates OCa progression [20,21]. Plus, it is shown that IL-1β, IL-6, and TNF-α formed by activated immune agents and/or the tumor itself, induce the growth of cancer cells and affect the prognosis and clinical status of the disease via increasing resistance to chemotherapy and stimulating symptoms (e.g., weight loss, anemia, depression, and anorexia) [22]. Oxidative stress, namely reactive nitrogen species (RNS) and reactive oxygen species (ROS), are another factor involved in many pathological conditions, such as OCa, by genetic instability enhancement, angiogenesis promotion, and abnormality in cell proliferation [23,24]. Exogenous agents, like hypoxia, infection, and chronic inflammation, are among the main sources of oxidative stress [25]. Mounting evidence has demonstrated that ROS can modulate the biogenesis and expression of microRNAs by epigenetic alterations, regulating biogenesis course, and transcription factors [26]. MicroRNAs, as noncoding RNAs, have a role in chemoresistance, carcinogenesis, proliferation, apoptosis, cell cycle, invasion, and metastasis. Impairments of microRNAs can lead to the onset and progression of OCa [27]. Possibly, the most important feature of any cancer is genetic changes that mediate the development and progression of tumors [28]. In this line, it is revealed that the presence of mutations in PTEN (Phosphatase and tensin homolog), P53, BRCA(Breast Cancer)1, and BRCA2 genes, and tumor suppressor factors can develop OCa (Figure 2) [29,30,31].
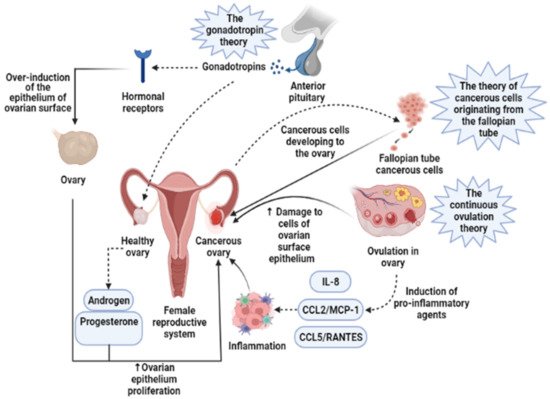
Figure 1. Three main theories regarding the development of ovarian cancer are based on induction of the epithelium of ovarian surface by hormonal receptors, increased induction of pro-inflammatory agents during continuous ovulation, and cancerous cells originating from the fallopian tube. IL-8, Interleukin-8; CCL2/MCP-1, Monocyte chemoattractant protein-1; CCL5/RANTES, CC Chemokine Ligand-5.
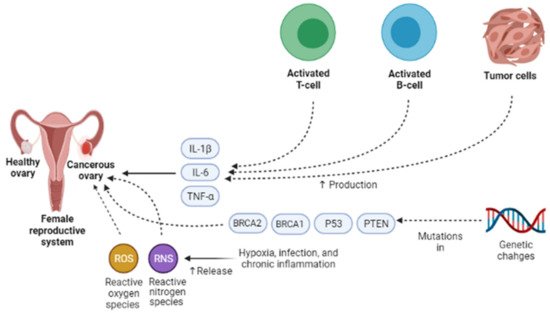
Figure 2. Different endogenous and exogenous factors modify the development and prognosis of ovarian cancer. IL-1β, IL-6, and TNF-α accelerate the growth of cancer cells and affect the prognosis and clinical status of the disease via increasing resistance to chemotherapy and stimulating symptoms. Exogenous factors, such as hypoxia, infection, and chronic inflammation, are the main sources of oxidative stress, namely reactive nitrogen species (RNS) and reactive oxygen species (ROS). They can contribute to ovarian cancer development via genetic instability enhancement, angiogenesis promotion, and abnormality in cell proliferation. One of the most important features of ovarian cancer is genetic changes that mediate the development and progression of tumors. In ovarian cancer, the presence of mutations in PTEN, P53, BRCA1, and BRCA2 genes, tumor suppressor factors, can lead to ovarian cancer development. IL-1β, Interleukin-1β; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor α; PTEN, Phosphatase and tensin homolog; BRCA1, Breast cancer type 1; BRCA2, Breast cancer type 1.
3. Use of Curcumin and Its Nanoformulations against Ovarian Cancer
Curcumin (CUR) is characterized as a yellow and hydrophobic herbal component that is originated from the turmeric plant (Curcuma longa L. Zingiberaceae) [32]. Growing evidence has shown some positive effects of CUR in medicine, such as anti-tumor, anti-inflammatory, anti-oxidative, immunoregulatory, anti-fungus, and anti-bacterial features [33,34], such as breast, ovarian, prostate, gastric, colorectal, pancreatic, and cervical cancers [35,36,37,38]. Regarding anti-cancer mechanisms of CUR, it has to be said that several signaling pathways are affected by that, for example, JAK (Janus Activated Kinase)/STAT (signal transducer and activator), PI3K (Phosphoinositide 3-kinases)/Akt, MAPK (mitogen-activated protein kinase), NF-ĸB, p53, Wnt/β-catenin, and apoptosis-related signaling (Figure 3). Furthermore, CUR can suppress epithelial-mesenchymal transition (EMT), angiogenesis, proliferation, metastasis, and invasion of the tumor by modulating the expression of non-coding RNA (ncRNA) associated with the tumor [39,40,41,42]. In the study of Shi et al., it was revealed that CUR can considerably suppress the growth and stimulate apoptosis in human OCa cell line Ho-8910. In their research, the use of 40 μM CUR caused a reduction in pro-caspase-3, Bcl-XL, and Bcl-2, whereas Bax and p53 levels were elevated in the treated cells with CUR [38]. Triggering AMP-activated protein kinase (AMPK), which stimulates cell apoptosis and inhibits cell proliferation in several cancers, in a p38-dependent way is another mechanism of CUR action in ovarian cancer cells CaOV3 [43]. In an animal investigation on OCa, it was manifested that CUR can dramatically suppresses STAT3 and NF-ĸB signaling pathways [44]. Liu et al. have pointed out that CUR can stimulate human OCa cell autophagy through AKT/mTOR (mammalian target of rapamycin)/p70S6K pathway suppression [45]. Despite these, the clinical application of CUR has been limited owing to its instability and low water solubility, which in turn give rise to poor bioavailability of CUR in cancerous cells. Attempts toward elevating the therapeutic effectiveness of Cur have been carried out through various techniques [46]. An in vivo and in vitro investigation showed that nanocurcumin in combination with cisplatin, a common treatment for OCa, could lead to a remarked reduction of the weight and volume of ovarian tumors. In addition, this treatment decreased PI3K, JAK, TGF-β, Ki67 expression, and Akt phosphorylation [51]. Hu et al. (2020) demonstrated that the use of Docetaxel curcumin/methoxy poly (ethylene glycol)-poly (L-lactic acid) (MPEG-PLA) copolymers nanomicelles cause the Suppression of tumor proliferation and angiogenesis (Table 1). The study of Bondi et al. (2017) concluded that biocompatible Lipid nanoparticles as carriers improved curcumin efficacy in ovarian cancer treatment and caused the Reduction of cell colony survival, inhibition of tumor growth, and apoptosis induction (Table 1). Dendrosomal nano-curcumin caused the reduction of cancer cell viability, a decrease of LncRNAs expression of H19 and HOTAIR, and an increase in the expression of MEG3 LncRNA and Bcl2 protein (Table 1). The study of Sandhiutami et al. (2021) showed that co-use of curcumin nanoparticles and Cisplatin caused the Decrease of ovarian tumor weight and volume, reduction of PI3K, TGF-β, JAK, and Ki67 expression, Akt and STAT3 phosphorylation, and decrease of IL-6 level (Table 1). In general, Curcumin is an efficient agent with anti-tumor, antioxidant, and anti-inflammatory activities. The main mechanisms of action by which curcumin exhibits its unique anticancer activity include inducing apoptosis and inhibiting proliferation and invasion of tumors by suppressing a variety of cellular signaling pathways.
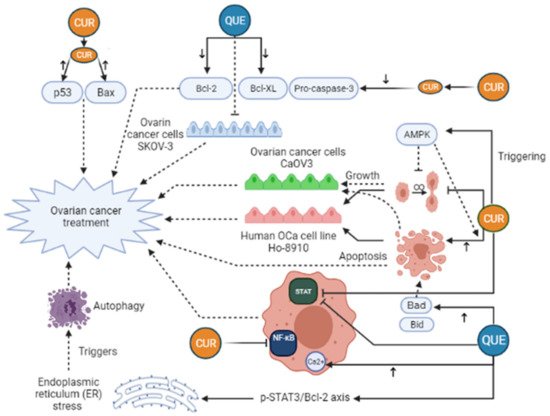
Figure 3. Curcumin (CUR) and Quercetin (Que) can exert an anti-cancerous effect on ovarian cancer in many different pathways. CUR triggers AMP-activated protein kinase (AMPK) that leads to stimulation of cell apoptosis and inhibition of cell proliferation. Moreover, CUR can decrease pro-caspase-3, Bcl-XL, and Bcl-2 levels, whereas Bax and p53 levels rise in the treated cells with CUR. These changes lead to ovarian cancer treatment. Furthermore, CUR can exert a significant inhibitory effect on STAT3 and NF-ĸB signaling pathways. Quercetin (Que) can modify many pathways and play a role in ovarian cancer treatment. Que decreases the anti-apoptotic agents, like Bcl-2, Bcl-xL, while it elevates the expression of pro-apoptotic agents, such as Bad and Bid, leading to increased apoptosis and ovarian cancer treatment. In addition, the elevation of cytosolic Ca2+ levels due to Que consumption can take part in ovarian cancer treatment. Que triggers autophagy by endoplasmic reticulum (ER) stress by the p-STAT3/Bcl-2 axis as well. Bcl-XL, B-cell lymphoma-extra-large; BAX, BCL2-associated X protein; Bcl-2, B-cell lymphoma 2; Bad, BCL2 associated agonist of cell death; Bid, BH3-interacting domain death agonist; STAT, Signal transducer and activator of transcription; NF-ĸB, Nuclear factor-kappaB.
Table 1. Nano-based formulations of curcumin, quercetin, and resveratrol through various mechanisms affect ovarian cancer.
| Type of Nano-Based Herbal Formulation | Mechanism/Effect | In Vivo/In Vitro | References |
|---|---|---|---|
| PLGA-phospholipid-PEG nanoparticles comprising curcumin | Downregulation of P-glycoprotein | In vitro | [52] |
| Niosome-encapsulated curcumin | Arresting the cell cycle at the S phase and apoptosis induction | In vitro | [49] |
| Docetaxel curcumin/methoxy poly (ethylene glycol)- poly (L-lactic acid) (MPEG-PLA) copolymers nanomicelles |
Suppression of tumor proliferation and angiogenesis | In vivo/in vitro | [53] |
| Curcumin—loaded nanostructured lipid carrier | Reduction of cell colony survival, inhibition of tumor growth, and apoptosis induction | In vitro | [54] |
| Gemini curcumin | Apoptosis induction | In vitro | [55] |
| Curcumin and paclitaxel co-delivery by hyaluronic acid-modified drug-loaded polyethylenimine and stearic acid | Downregulation of P-glycoprotein, and suppression of tumor cell migration | In vivo/in vitro | [56] |
| Dendrosomal nano-curcumin | Reduction of cancer cell viability, decease of LncRNAs expression of H19 and HOTAIR, and increase in the expression of MEG3 LncRNA and Bcl2 protein | In vitro | [57] |
| Co-use of curcumin nanoparticles and Cisplatin | Decrease of ovarian tumor weight and volume, reduction of PI3K, TGF-β, JAK, and Ki67 expression, Akt and STAT3 phosphorylation, and decrease of IL-6 level | In vivo/in vitro | [51] |
| Encapsulated quercetin into monomethoxy poly (ethylene glycol)- poly (3-caprolactone) |
Apoptosis induction and the suppression of angiogenesis | In vivo/in vitro | [58] |
| Encapsulated quercetin into methoxypoly(ethylene glycol) Poly(caprolactone) | Apoptosis induction and cell growth suppression | In vivo/in vitro | [59] |
| PEGylated liposomal quercetin | Apoptosis induction, cell proliferation inhibition, and arresting the cell cycle at G0/G1 and G2/M phases | In vivo/in vitro | [60] |
| Resveratrol—ZnO nanohybrid | Mitochondrial membrane depolarization and ROS formation | In vitro | [61] |
| RGD-conjugated Resveratrol human serum albumin nanoparticles | Reduction of cell viability and tumor growth inhibition | In vivo/in vitro | [62] |
| Resveratrol—bovine serum albumin nanoparticles |
Reduction of cancer cell growth, activation of cytochrome C, upregulation of caspase-3 and caspase-3 expression |
In vivo/in vitro | [63] |
This entry is adapted from the peer-reviewed paper 10.3390/ph14121315
This entry is offline, you can click here to edit this entry!
