3. New Technologies Herald a New Dawn—The Ketolactonases Reveal Their True Identity
Perhaps based on the false premise that most if not all of the fundamental aspects of both the structure and function of the DKCMOs of
P. putida ATCC 17453 had been resolved, active interest in these enzymes was marginalised until a new facet of investigation was initiated at Exeter University in the early 1990s. This resulted from the realisation that the highly enantiospecific ketolactonases could be deployed as successful biocatalysts to biotransform both natural and xenobiotic ketones thereby generating products with multiple chiral centres of considerable value for chemoenzymatic synthesis [
57, 58, 61-63]. Although evidently successful when deployed using the comparatively small-scale protocols developed at the University of Exeter, it was obvious that the potential to scale up the technology for larger industrial-scale applications would be significantly hindered by reliance on the then established purification protocols, which were both protracted and FPLC-based, and as a consequence, low-yielding [
34,
62]. This realisation then promoted a new initiative led by Professor Uwe Bornscheuer at Greifswald University to develop suitable vectors to exploit host recombinant expression of the relevant ketolactonase genes as a potential way to generate larger amounts of each biocatalyst. As the ketolactonases were still considered to be true flavoproteins at the time, a successful outcome from the initiative would require both the identification of a suitable complementary NADH-dependent redox partner and the development of an effective coupled-enzyme operating system. Initial studies [
55] were focussed on 2,5-diketocamphane 1,2-monooxygenase, for which it was possible to isolate a relevant gene from DNA sourced from the CAM plasmid of
P. putida ATCC 17453 with the aid of primers derived from GenBank entry AY450285.1 (2003), The gene, now known to correspond to
camE25-1, was then used to generate relatively large amounts of the corresponding protein by exploiting the well-developed
E. coli BL21(DE3) recombinant expression system. Contrary to expectations, although only the gene coding for the oxygenating moiety of 2,5-DKCMO was expressed in the heterologous host, significant lactonizing activity was recorded with partially purified preparations of the recovered recombinant enzyme, which was established to be flavin-free. Attention then turned to the enantiocomplementary 3,6-DKCMO [
56], an initiative facilitated by deploying gene walking PCR from
camE25-1 to successfully identify
camE36, the corresponding gene on the CAM plasmid that codes for 3,6-diketocamphane 1,6-monooxygenase (vide infra). Subsequent heterologous expression of
camE36 the in the same host again resulted in the unexpected outcome of significant lactonizing activity being recorded with partially purified flavin-free preparations of the recovered recombinant enzyme. These data were interpreted by Bornscheuer as indicating that one or more native activities of the
E. coli host was serving to complement the cloned ketolactonases by generating FMNH
2, thereby resulting in the recorded lactonizing activity of both the recombinantly expressed enzymes.
It was these purely serendipitous outcomes of the recombinant expression studies at Greifswald University that prompted the initial realisation that almost half a century of dogma, built on foundations initially promulgated by Irwin Gunsalus at Illinois University, and subsequently reiterated by Peter Trudgill at Aberystwyth University, could actually be wrong. Thus, the Greifswald University data suggested that the ketolactonases of camphor-grown
P. putida ATCC 17453, rather than being true flavoproteins, may instead be fd-TCMOs, as such corresponding to a few other recently reported bacterial enzymes acknowledged as being a completely novel class of monooxygenases [
2]. Undoubtedly the best characterised bacterial fd-TCMOs at that time were the luciferases from
Vibrio harveyi,
V. fischeri,
Photobacterium phosphoreum,
P. legionathi, and
Xenorhahdus luminescens [
65].The fact that luciferases, like the ketolactonases, are enzymes that function as Baeyer–Villiger monooxygenases was considered by Bornscheuer as indirect support for his radical proposal, as was the fact that bacterial luciferases and the ketolactonases had been grouped together over a decade previously, as so-called Type II BVMOs, on the basis of a number of other shared structural and functional properties [
31]. In an attempt to further investigate and characterise the
E. coli-triggered lactonizing activities of the overexpressed ketolactonases from
P. putida ATCC 17453, a dual initiative was then launched [
66,
67] to identify both the nature of the competent FMNH
2-generating enzyme(s) in
E. coli, and any equivalent enzyme(s) in
P. putida ATCC 17453 that would correspond to
E1 (vide supra), Trudgill’s apocryphal monomeric 36 kDa dehydrogenase [
34,
40,
41,
54], ironically an activity that was transiently referred to over 50 years previously as ‘FMN reductase’ [
30]. The functional native
E. coli activity was successfully identified as the known monomeric 26.2 kDa FMN-dependent enzyme Fre, which can serve a dual purpose ferric-flavin reductase role [
68]. However, while a number of putative flavin reductase genes were identified in the genome of
P. putida ATCC 17453 by CODEHOP PCR [
69], including a 28.5 kDa enzyme with 83% sequence homology to the ferric-flavin reductase FprB from
P. putida KT2440, ultimately the latter programme of research was unsuccessful because none of the identified candidate reductase genes yielded any equivalent titres of active protein when deployed in the
E. coli BL21(DE3) recombinant expression system [
66]. Consequently, further interest in the programme subsequently declined at Greifswald University, and irrefutable confirmation both of the status of the ketolactonases of
P. putida ATCC 17453 as fd-TCMOs, and identification and characterisation of the relevant native flavin reductases then had to await subsequent inputs, initially by Iwaki et al. [
44], and then more comprehensively by Willetts and Kelly [
47,
70].
The definitive evidence that the ketolactonases from camphor-grown
P. putida ATCC 17453 are indeed FMNH
2-dependent Class C TCMOs was provided by a joint initiative led by Professors Hiroaki Iwaki and Yoshie Hasegawa (Kansai University), and Peter Lau (McGill University). Their seminal paper (Iwaki et al. [
44]) published in May 2013 describes the isolation and full characterisation of a 37 kDa FMNH
2-donating enzyme present in cells of the bacterium harvested in the late log-early stationary phase of growth on (+)-camphor, and confirmed that it could facilitate efficient lactonization reactions catalysed by purified preparations of both ketolactonases. These characteristics of the enzyme, which was given the trivial name Fred (Flavin reductase), make it a strong candidate both for the activity in
P. putida ATCC 17453 serendipitously predicted by Bornscheuer [
54,
55], and for the enzyme that had been misidentified throughout both Gunsalus’s and Trudgill’s prior studies (vide supra) as being a monomeric 36 kDa protein variously referred to as FMN reductase [
30],
E1 [
36], NADH oxidase [
37,
54], and NADH dehydrogenase [
34,
40,
41]. Purified samples of Fred prepared from late log-early stationary phase cells grown on (+)-camphor were sequenced, and that data then used to design probes that confirmed that the corresponding gene was located on the chromosomal DNA rather than the CAM plasmid. Analysis of the gene predicted a corresponding protein product of 170 amino acids, including the presence of the characteristic flavin reductase motifs GDH [
71] and YGG [
72]. The predicted
Mr of the protein was 18,466. Unlike the equivalent chromosomal gene coding for a 28.5 kDa reductase identified by the earlier research undertaken at the Greifswald University [
66], subsequent cloning and over expression of the gene coding for Fred generated large quantities of fully functional protein, enabling the enzyme to be highly purified and then extensively characterised. These studies established that the active form of the enzyme was an NADH-specific 2 × 18.5 kDa homodimer. The data from relevant kinetic analysis of purified samples of enzyme confirmed that while FMN, FAD and Rf can all serve as substrates, FMN was the flavin most efficiently reduced by Fred. Collectively, these outcomes confirm that Fred corresponds to a Class II non-flavoprotein reductase [
3], which operate a sequential rather than a ping-pong reaction mechanism [
2,
4] to transfer generated FMNH
2 to serve as a cosubstrate for redox partner enzymes by rapid free diffusion. Comparison with protein databases confirmed that Fred appears to most closely resemble NTA-MoB, an established flavin reductase partner of nitrilotriacetate monooxygenase from
Chelatobacter heintzii ATCC 29,600 [
73] which was one of the first FMNH
2-dependent TCMOs to be identified in the mid-1990s.
As well as their seminal contribution by confirming for the first time the true nature of the ketolactonases of
P. putida ATCC 17453 as fd-TCMOs, the outcomes from Iwaki et al.’s research [
44] also significantly increased knowledge both about the CAM plasmid as a whole, and the specific concatenated
cam operon region which codes for all the enzymes of the camphor degradation pathway. One novel notable outcome was that contrary to earlier predictions [
74,
75], the plasmid proved to be a large linear 533 kb double-stranded transmissible genetic element, thereby updating its previously perceived circular nature. Their use of a combination of standard cloning and sequencing techniques to search for individual genes also proved rewarding (
Figure 7). A corresponding gene for the oxygenating moiety of 3,6-diketocamphane 1,6-monooxygenase (
camE36 [
orf19]) was located within a contiguous 40.5 kb region of the established
camRDCAB locus of the CAM plasmid, confirming an earlier relevant study at the Greifswald University [
56]. However, by way of contrast, the equivalent search that focused on 2,5-diketocamphane 1,2-monooxygenase yielded an unexpected novel outcome by identifying two separately located corresponding genes which were designated
camE25-1 (
orf4, coding for 2,5-DKCMO-1), and
camE25-2 (
orf22, coding for 2,5-DKCMO-2). Thus, for the first time, it became apparent that 2,5-DKCMO, which had historically been considered to be a single protein entity, actually comprised two distinct isoenzymic forms of the same enzyme. This novel outcome then raised the possibility that the detection in 1986 by Taylor and Trudgill [
54] of two electrophoretically separable forms of this enzyme (A and B), could have represented the first, albeit unrecognised, evidence of the isoenzymic status of 2,5-DKCMO, rather than corresponding to two different post-translationally modified forms of a single protein as proposed at the time. As such it represents another example of how historical misinterpretation of data has dogged the ketolactonases for several decades.
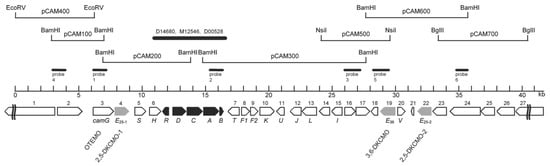
Figure 7. Localization of additional genes and predicted open reading frames (ORFs) flanking the established initial genes of the camphor
camDCAB operon and its repressor,
camR, on an~40.5-kb established sequenced region of the CAM plasmid of
P. putida ATCC 17453. The predicted ORFs or genes are sequenced numbered from 1 to 27, except for the established
camRDCAB genes, which are shaded in black. The numbered from 1 to 27, except for the established
camRDCAB genes, which are shaded in black. The orientation of the arrows indicates the direction of gene transcription. The candidate genes of this study (
camE25–1,
camE25–2, and
camE36) representing the three diketocamphane monooxygenase (DKCMO) isozymes are highlighted in grey. The previously established OTEMO-encoding gene been designated
camG in accordance with the respective catabolic step. Figure reproduced from Iwaki et al. [
44] with permission. American Society for Microbiology, licence number 4487631509592.
A number of direct and indirect consequences subsequently resulted from Iwaki et al.’s seminal 2013 studies. An obvious direct consequence was that the identification and sequencing of the
camE25-1,
camE25-2, and
camE36 genes presented the first opportunity to compare the complete primary structure of the ketolactonases induced in camphor-grown
P. putida ATCC 17,453. Translation of the relevant nucleotide sequences confirmed that 3,6-diketocamphane 1,6-monooxygenase is the larger protein. It comprises 378 amino acid residues compared to the 363 amino acid residues that characterise the two proteins coded for by the
camE25-1 and
camE25-2 genes. This outcome served to correct a further example of the misinterpretations of data that are prevalent throughout the history of characterising camphor metabolism by
P. putida ATCC 17453, namely the proposal made 20 years previously by Jones et al. [
34] that 3,6-DKCMO is the smaller protein. Rather than using direct corresponding gene sequence data, Jones et al. had reached their earlier conclusion indirectly by comparing the sedimentation characteristics of purified preparations of both ketolactonases when subjected to density gradient ultracentrifugation, which clearly had yielded a less reliable outcome. Further in-depth analysis of the sequence data (
Figure 8) using standard alignment tools has served to emphasise the considerable similarity between all the three sequences. While the two isoenzymic 2,5-diketocamphane 1,2-mono-oxygenases exhibit the highest alignment score (1852 using BLOSUM62), the equivalent scores for both isoenzymes when compared to 3,6-diketocamphane 1,6-monooxygenase (961 and 965) are very similar. The closely related nucleotide sequences of the
camE25-1,
camE25-2, and
camE36 genes suggests that they probably arose by gene duplication and subsequent divergence [
76]. That being the case, an interesting future study would be to compare the outcomes of the resolved natural evolutionary relationships between 2,5-DKCMO-1, 2,5-DKCMO-2, and 3,6-DKCMO, and a multi-round programme of directed evolution of the corresponding genes [
77].
Figure 8. Protein sequence alignment of the oxygenating subunits of 2,5-DKCMO-1 (orf-4, camE25-,) 2,5-DKCMO-2 (orf-22, camE25-2) and 3,6-DKCMO (orf-19, camE36). Amino acid residues: common to 2,5-DKCMO-1, 2,5-DKCMO-2, and 3,6-DKCMO = black; common to 2,5-DKCMO-1 and 2,5-DKCM-2 = magenta; exclusive to 2,5-DKCMO-1 = green; exclusive to 2,5-DKCMO-2 = red; exclusive to 3,6-DKCMO = blue.
An indirect consequence of Iwaki et al.’s pioneering 2013 studies [
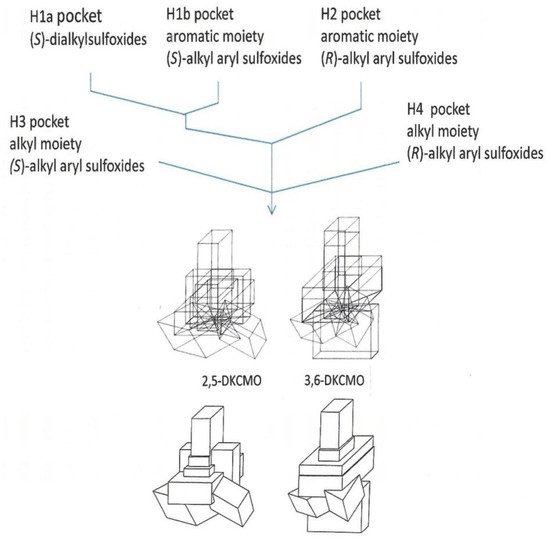
44] was that the resultant knowledge of the primary structures of all the DKCMO isoenzymes resparked interest in determining their corresponding three-dimensional structures. Historically, surprisingly little relevant information on structural features of the isoenzymic ketolactonases had been published. The one exception was a 1998 study [
59] that deployed so-called ‘cubic space models’ [
78] to develop three-dimensional (3D) representations of the active sites of the enantiocomplementary DKCMOs based on the outcomes of biotransformation conducted with twenty-three organosulphide substrates (
Figure 9). This study had its strengths and weaknesses. With hindsight, it has to be appreciated that the representation generated for 2,5-DKCMO represent a collage of the outcomes from 2,5-DKCMO-1 and 2,5-DKCMO-2. However, the study has significant merit because the DKCMOs, like other enzymes, undergo sequential structural changes during catalysis [
79], and a strength of this approach is that the resulting models reflect the relative extent of relevant dynamic structural changes for the DKCMOs. It was also clear from this 1998 study that the substrate-binding active site of 3,6-DKCMO was significantly larger than that of 2,5-DKCMO, which may correspond with 3,6-DKCMO being confirmed to be the larger protein by Iwaki et al.’s study [
44].
Figure 9. Rationale for superimposing the minimalised structures of the sulfoxides formed by 2,5-DKCMO and 3,6-DKCMO, and the resultant total dimensions (‘cubic space’) of the relevant enzyme active sites.
Iwaki et al.’s intimate knowledge of the CAM plasmid and relevant recombinant expression protocols able to deliver the large amounts of DKCMO protein that would be necessary to generate diffraction-grade crystals resulted in them being recruited into a collaborative X-ray crystallographic study with Professor Jennifer Littlechild and Dr Michail Isupov Exeter University) to determine the 3D structure of 3,6-diketocamphane 1,6-monooxygenase, an undertaking that ultimately proved to be controversial [
60]. The benchmark for such studies of the O
2-dependent enzymes of camphor-grown
P. putida ATCC17453 had been set a number of years earlier by Thomas Poulos’s high-level resolution of the crystal structure of camphor 5-monooxygenase [
80]. Capitalising on experience gained from a series of unsuccessful attempts previously undertaken with 3,6-DKCMO [
81,
82,
83], Isupov et al. used the known structure of the α-subunit of the luciferase from
Vibrio harveyi in combination with molecular replacement and MAD phasing techniques and relevant density modification to generate a crystal structure of the ketolactonase (PDB code 4UWM [
60]). Although the choice of the α-subunit of the luciferase from
Vibrio harveyi to resolve the phase problem represented the best available sequence homology (16%) that could be sourced at the time [
84], this is well within the so-called ‘twilight zone of protein sequence alignments’ which make comparative structure predictions unreliable [
85]. Further, it was known that the two proteins exhibit some significant structural and functional differences [
86,
87], which may in part explain why Isupov et al. reported that their attempts to model active site binding of a flavin moiety proved to be problematical. Additionally relevant in this respect is that the modelling studies were undertaken with FMN, a flavin for which functional binding is only relevant for true flavoproteins where FMN serves as a tightly-bound cofactor. For 3,6-DKCMO, convincingly shown to be an FNMH
2-dependent TCMO in almost two years previously by Iwaki et al. [
44], the relevant flavin moiety is performed FMNH
2. which functions as a fully reduced redox flux cosubstrate, generated by one or more competent partner flavin reductases (vide infra). Despite Iwaki, Hasegawa and Lau being cited as coauthors, this misconception regarding the correct nature of the relevant flavin moiety for 3,6-DKCMO is reflected in Isupov et al. [
60] by the multiple inclusion of the term ‘cofactor’ which contrasts with a total absence of the term ‘cosubstrate’. Additionally relevant in this context are both the well characterised difference in three-dimensional shape between FMNH
2 and FMN [
87,
88], and the fact that FMN exhibits random binding to fd-TCMOs [
2], as evidenced specifically for 3,6-DKCMO by a kinetic study which confirmed a 500-fold difference in the binding capacities of FMNH
2 and FMN [
47]. Consequently, another flawed chapter has been added to the history book for the ketolactonases, and a revision must await the outcome of a future study based a combination of factors. Firstly, the use of a closer protein match than the α-subunit of luciferase to resolve the phase problem, secondly a correct understanding of the role of FMNH
2 in the generation and deployment of ‘active oxygen’ by the DKCMOs, and thirdly an appreciation of the functional domain movements that characterise the mode of action of BVMOs [
89].
There was one final bizarre twist to this multi-national collaborative study of 3,6-DKCMO that is directly related to the extensive knowledge Iwaki, Hasegawa and Lau had gained from their prior investigation [
44] of camphor-grown
P. putida ATCC 17453. Significantly, their 2013 study reported the complete nucleotide sequence of a concatenated 40.5 kb operon on the CAM plasmid that coded for all the degradation pathway enzymes necessary to catabolise the C10 terpene via OTE and Δ
2-5-3,4,4-trimethylpimelyl-CoA to the key central intermediary metabolites acetyl-CoA and isobutyl-CoA (
Figure 2). However, another important outcome of their 2013 study was that the sole redox partner identified as providing the requisite FMNH
2 cosubstrate for the DKCMO isoenzymes to function as fd-TCMOs was the chromosome-coded flavin reductase Fred. This exception is important because, taken at face value, Iwaki et al.’s results imply that the CAM plasmid of
P. putida ATCC 17453 cannot function as a fully autonomous metabolic entity (vide infra), making it a notable exception to other then known bacterial catabolic plasmids [
8,
75,
90]. This perceived deficiency then promoted Littlechild and Isupov, the two cited lead authors of the 2015 study of CAM plasmid-coded 3,6-DKCMO [
60], to include an unsupported claim to have ‘now identified a flavin reductase adjacent to the 3,6-DKCMO gene on the CAM plasmid’. However, the claim by Littlechild and Isupov was clearly fallacious given that the complete sequence of the 40.5 kb concatenated region of the CAM plasmid, including the gene coding for 3,6-DKCMO (
orf19) plus all 18 upstream and 7 downstream proximal
orfs, had been published 30 months previously by Iwaki, Hasegawa and Lau [
44], all of whom are inexplicably cited coauthors of the 2015 study. Fortunately this was one corruption of the DKCMO database that was quickly investigated by the publishing journal, and subsequently corrected by a relevant corrigendum that unreservedly withdrew the unsupported claim and replaced it with the relevant cognisant information [
60].
Resolving the issue of the self-sufficiency of the CAM plasmid did, however, trigger a broader based research initiative to investigate factors that control the supply of the FMNH
2 cosubstrate necessary for the DKCMOs to function effectively as fd-TCMOs. The initial objective of the study was to identify which, if any, indigenous reductases of
P. putida ATCC 17453 other than Fred can support the ketolactonases [
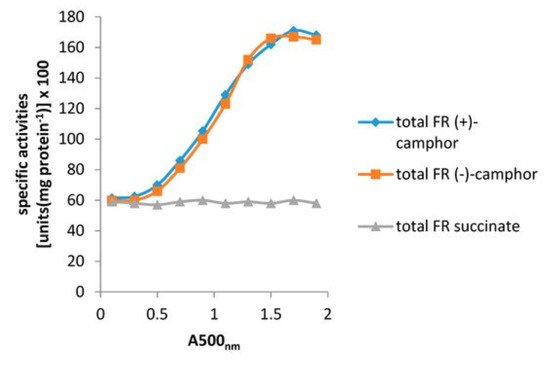
70]. Studies of total flavin reductase activity throughout the growth of
P. putida ATCC 17453 on either succinate- or (+)- or (−)-camphor-based minimal media confirmed that very similar titres of activity were detectible in the earliest lag phase cells sampled after inoculation into all three media. However, whereas that level remained remarkably consistent in succinate-grown cells throughout trophophasic growth, the flavin reductase titre of cells grown on either (+)- or (−)-camphor-based minimal medium was progressively induced to a maximum 2.8-fold higher level during late trophophasic growth before plateauing after entry into the stationary (idiophasic) phase of growth (
Figure 10). This data implied that growth of
P. putida ATCC 17453 on either enantiomer of camphor generated an equivalent enhanced intracellular demand for FMNH
2. A combination of gel-filtration chromatography, electrophoresis, and comparative sequence data analysis of purified recovered samples confirmed that two monomeric reductases (Frp1, 27.5 kDa and Frp2, 28.5 kDa) which correspond with the previously characterised chromosome-coded ferric-flavin reductases FprA and FprB from
P. putida KT2440 [
91], were the only relevant activities recorded after growth on succinate. Based solely on relevant MW data, it is possible that Frp2 corresponds to the same previously uncharacterised flavin reductase-type gene detected in the course of the prior CODEHOP PCR studies carried out at the University of Greifswald [
66]. Equivalent analysis of the camphor-grown cells confirmed that very similar constitutive titres of Frp1 and Frp2 were present after growth on either camphor enantiomer.
Figure 10. Specific activity of total flavin reductase (FR) throughout the growth of P. putida ATCC 17543 on either (+)-camphor, (−)-camphor, or succinate as sole carbon source.
Attention then focused on what other activities were responsible for the significantly up-regulated total camphor-induced reductase activity. This search then identified two principal additional camphor-induced reductase activities, both induced by growth on either terpene enantiomer. Perhaps not unsurprisingly, one of these was confirmed to be Fred, the 37 kDa homodimer reported previously as the sole reductase activity of
P. putida ATCC 17453 [
44]. More unexpectedly, however, was that the purified monomeric protein responsible for the second additional camphor-induced flavin reductase activity had an N-terminal amino acid sequence consistent with that of putidaredoxin reductase (PdR), a 48.5 kDa protein coded for by the
camA gene of the CAM plasmid, and known to serve in the role of an FAD reductase as one of the functioning components of camphor 5- monooxygenase [
92], but which had not been reported previously to serve as an FMN reductase. Interestingly, the initiation codon GTG for
camA is a rare start codon, and is thought to be important in the control of PdR abundance. Purified samples of Fred, Fpr1, Fpr2, and most significantly PdR, were then confirmed to be equally active in supporting lactonization reactions with a high degree of enantioselectivity by purified preparations of both DKCMOs. This previously unrecognised ability of PdR to serve a functional role in the redox flux that supports the DKCMOs has significant implications for the metabolic autonomy of the catabolic CAM plasmid of
P. putida ATCC 17453 (vide infra). That four different identified reductases present in camphor-grown
P. putida ATCC 17453 can serve effectively as distal sources of the requisite FMNH
2 for both of the DKCMOs confirms the non-specific nature these redox relationships, as is a characteristic feature of fd-TCMOs [
2]. This catholic relationship has been emphasised further by the recent demonstration that biomimetic nicotinamide analogues serving as hydride donors can participate in reductase-free FMNH
2 generating systems able to support the biooxygenating activity of highly purified 2,5-DKCMO [
93].
The relative roles of the each of the identified flavin reductase activities in supporting lactonizing activity by the DKCMOs were then examined in more detail in a subsequent study [
47], which both logged the titres of all relevant reductase and oxygenating activities promoted by (
rac)-camphor as a growth substrate, and additionally characterised relevant kinetic data for the generation of FMNH
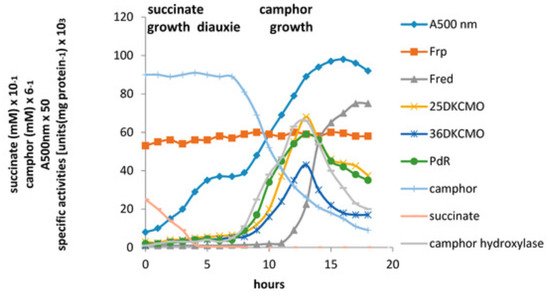
2 and its subsequent transfer to the oxygenating subunits of the DKCMOs. The camphor-promoted events were monitored (
Figure 11) by following up on the early observation by Gunsalus et al. [
42] that during the diauxic growth of
P. putida ATCC 17453 on a defined succinate plus (
rac)-camphor medium, the enzymes of the camphor degradation pathway were only expressed after complete exhaustion of the succinate. With respect to the oxygenating activities, both the timing and extent of induction of 2,5-diketocamphane 1,2-monooxygenase and camphor 5-monooxygenase were very similar in response to the swop to (
rac)-camphor as the growth substrate. While the enantiocomplementary 3,6-diketocamphane 1,6-monooxygenase followed a very similar time course, the level of induction was consistently lower, confirming some earlier preliminary observations [
57,
62,
63]. Not unexpectedly, the profile of induction of PdR activity coincided exactly with that of the composite camphor 5-monooxygenase, of which the monomeric 48.5 kDa protein is a confirmed functional subunit [
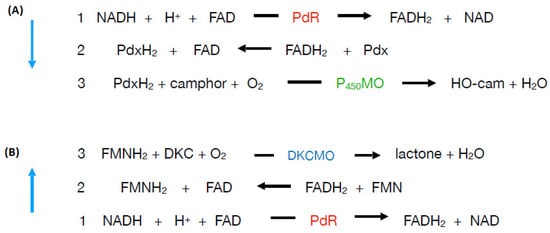
94]. Kinetic data derived from characteristic changes in the relevant absorption spectra confirmed conclusively that the highly purified PdR preparation accepted reducing power exclusively from NADH thereby generating bound FADH
2, which in turn was able to pass reducing power to unbound FMN added subsequently as a cosubstrate. This sequential transfer of reducing power from NADH via FADH
2 to FMNH
2 defines a de novo role for PdR, and explains the confirmed ability of a highly purified preparation of the enzyme to function as the effective redox partner for highly purified preparations of both enantioselective ketolactonases [
47]. In turn, this is key evidence that the catabolic CAM plasmid of
P. putida ATCC 17453 can serve as an autonomous metabolic entity (vide infra).
Figure 11. Changes in the optical density (A500nm), succinate (mM), (rac)-camphor (mM), and the specific activity of key enzymes of (rac)-camphor degradation during diauxic growth of by P. putida ATCC 17453 on succinate plus (rac)-camphor based defined medium. Camphor hydroxylase = camphor 5-monooxygenase.
Detailed investigation of Fred, the 37 kDa homodimeric flavin reductase which Iwaki et al. [
44] claimed corresponded to the activity variously referred to as
E1, FMN reductase, FMN-coupled NADH oxidase, or NAD dehydrogenase in early studies at Illinois [
30,
34,
36,
37,
40,
41,
54], confirmed that it is, like PdR, a camphor-induced enzyme. Initial Michaelis Menton kinetic studies using a highly purified preparation of the enzyme confirmed Iwaki et al.’s original conclusion that Fred can only effectively transfer reducing power from NADH to FMN and shows minimal activity in any combination with either NADPH or FAD or riboflavin. Double reciprocal kinetic plots were then deployed to provide a more detailed understanding of the enzyme, thus confirming that the enzyme is a EC 1.5.1.x-type Class II non-flavoprotein reductase [
3,
95] that generates FMNH
2 by a sequential reaction mechanism [
2,
4].
The titres of Frp1 and Frp2 were remarkably consistent throughout each stage of the progression from succinate-dependent to camphor-dependent growth, confirming the outcome of the earlier growth study [
47] which identified that both Frp1 and Frp2 are constitutively expressed activities in
P. putida ATCC 17453. Kinetic studies confirmed that a purified preparation of Frp1, while totally inactive with NADPH, was able to serve as an effective means of generating FMNH
2 from NADH while showing minimal and no corresponding activity with either FAD or riboflavin. While Frp2 gave similar outcomes, there was some limited generation of FMNH
2 from NADPH compared to NADH as an alternative source of reducing power. However, the relative kinetic data suggest that the recorded low level of activity with NADPH is unlikely to be physiologically significant. Double reciprocal plots of relevant kinetic data confirmed that both Frp1 and Frp2, like Fred, are EC 1.5.1.x-type Class II non-flavoprotein reductases [
3,
95], that generate FMNH
2 by a sequential reaction mechanism [
2,
4].
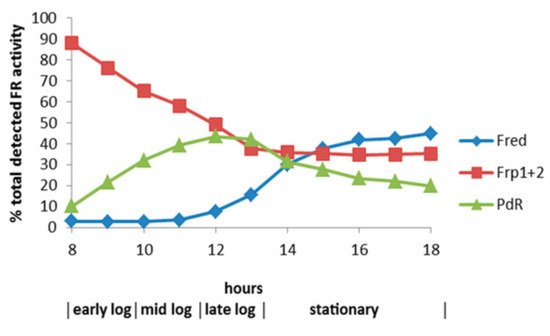
By reformatting the data from the diauxic growth study, a picture could be gained of the relative contribution of the different assayed FMNH
2-generating enzymes to the total flavin reductase activity titre throughout the progressive phases of the camphor-dependent growth of
P. putida ATCC 17453 (
Figure 12). The outcome confirmed the initially predominant importance of the combined constitutive Frp1 and Frp2 activities at the onset of camphor-dependent growth. However, this predominance was progressively diminished throughout early and mid log trophophasic growth by the steady incremental induction of the CAM plasmid-coded PdR activity. Thereafter, these three flavin reductase activities progressed to assume approximately equal importance during the late log phase in the growth cycle, before the relative importance of PdR declined significantly as induction of the protein is down-regulated after exit from trophophase into idiophase (stationary phase). Contrastingly, the titre of chromosome-coded camphor-induced Fred only began to assume any importance during the very late log phase of trophophasic growth, but then progressed to become the predominant FMNH
2-generating activity following entry into idiophase, a profile matched concomitantly by the declining titres of PdR and, as evident from
Figure 11, both DKCMOs. This time course for the up-regulation of Fred is more consistent with that of an enzyme involved in secondary (idiophasic) rather than primary (trophophasic) metabolism [
95]. It is relevant in this respect that species of
P. putida are known to produce a wide range of indigenous secondary metabolites [
96], including various polyketides [
97], for which fd-TCMOs are known to play important roles as ‘tailoring enzymes’ [
98,
99,
100].
Figure 12. Relative contribution of the different assayed FNR-generating enzymes to the total flavin reductase activity titre throughout the various phases of (rac)-camphor-dependent growth of P. putida reductase activity titre throughout the various phases of (rac)-camphor-dependent growth of P. putida.
Both the recorded growth substrate-dependent and growth phase-dependent changes in the titres of the inducible isoenzymic DKCMOs and their inducible flavin reductase partners Fred and PdR are indicative of elements of transcriptional control that may serve to up-regulate the pathway for the degradation of camphor in
P. putida ATCC 17453. The extent of such controls was then investigated using the antibiotics rifampicin and actinomycin D [
101]. This comprehensive study monitored the differential rates of synthesis of both chromosome-coded Fred and a number of CAM plasmid-coded activities including the ketolactonases in response to both camphor and key degradation pathway intermediates (
Figure 13). Augmented by the outcomes from some relevant earlier studies [
42,
47,
57,
70], the results confirmed that the genes that code for the enantioselective DKCMOs are subject to induction by the corresponding camphor enantiomer, along with the
camRDCAB polycistronic operon that codes for camphor 5-monooxygenase and 5-
exohydroxycamphor dehydrogenase. This coordinate transcriptional control of the first three successive steps of the catabolic pathway by the initial substrate represents ‘from the top’ coordinate pathway regulation, and has been reported in a number of other catabolic pathways in other
Pseudomonas spp. [
102,
103]. Further, the relevant differential rates of synthesis demonstrated that each enantioselective ketolactonase as well as being induced by its own corresponding diketone substrate was cross-induced by the complementary chiral diketocamphane from the opposite enantiomeric series, and additionally back-induced by OTE. These two interesting forms of induction confirm cross-inducibility and product induction, respectively, as two additional important elements of transcriptional regulatory control of the ketolactonases in camphor-grown
P. putida ATCC 17453.
Figure 13. Transcriptional controls of the pathway of (+)- and (−)-camphor degradation in P. putida ATCC 17453 ●+ = induction: −● = repression: A = camphor 5-monooxygenase (camCAB): B = exo-hydroxycamphor dehydrogenase (camD): C = 2,5-diketocamphane 1,2-monooxygenase (camE25-1 + camE25-2); D = 3,6-diketo- camphane 1,6-monooxygenase camE36); E = 2-oxo-△3-4,5,5-trimethylcyclo-pentenylacetyl-CoA synthetase (camF1 + F2); F = 2-oxo-△3-4,5,5-trimethylcyclo-pentenylacetyl-CoA monooxygenase (camG).
Product induction or so-called ‘from the bottom’ regulation is not unique to the DKCMOs of camphor-grown
P. putida ATCC 17453. It has been characterised in a number of other bacterial catabolic pathways [
104,
105,
106,
107,
108], and has been speculated to reflect the evolution of catabolic pathways ‘from the bottom to the top’ by the sequential acquisition of additional units of physiological function [
103,
104]. The confirmation of cross-inducibility of the DKCMOs by their complimentary chiral diketocamphane pathway intermediates supports earlier studies of the camphor degradation pathway which have consistently reported an equivalent significant element of cross-inducibility of both ketolactonases by each enantiomer of camphor [
47,
57,
62,
70]. The broad specificity of the relevant repressor proteins implicated by these various transcriptional controls, allied to the established patterns of coordinate induction has been suggested as a further example of a more general phenomenon characteristic of a number of different catabolic pathways of pseudomonads [
35,
42], the significance of which is to vest individual species with their acknowledged impressive metabolic versatility as illustrated by
P. putida KT2440 [
109]. Further light on this interesting aspect of the biochemistry of camphor-grown
P. putida ATCC 17453 would undoubtedly result from appropriate additional in depth transcriptomic analysis [
110]. These catholic patterns of transcriptional control of the isoenzymic DKCMOs contrast sharply with the established absolute stereoselectivity of the DKCMOs [
31,
34,
57,
58,
62]. Possible ramifications of this need to be considered in the broader context of other known characteristics of the camphor degradation pathway to the level of OTE (
Figure 2). In this respect, the known lack of stereoselectivity of both camphor 5-monooxygenase [
39] and 5-
exohydroxycamphor dehydrogenase [
111] is a significant factor, as is the unstable nature of the lactones formed by the enantiocomplementary DKCMOs which both spontaneously rearrange to the same achiral metabolite OTE. Consequently, any implied potential of
P. putida ATCC 17453 to discriminate between (+)- and (−)-camphor as a growth substrate by selectively up-regulating the corresponding enantioselective ketolactonase (
Figure 14) is negated by a combination of the established cross-inducibility of the relevant CAM plasmid genes by each of the enantiocomplementary camphor and diketocamphane isomers, and the multi-valent product induction of the genes by the shared degradation pathway intermediate OTE [
101]. The very similar nucleotide sequences of the
camE25-1,
camE25-2,, and
camE36 genes on the CAM plasmid (vide supra) suggests that they probably arose by gene duplication and subsequent divergence [
76], although any evolutionary advantage of the isoenzymic 2,5-DKCMOs relative to the implicit additional genetic load is difficult to imagine given the very low abundance of camphor as an available substrate in nature [
26]. Considered in a broader context, a comprehensive review has concluded that enantiocomplementary and duplicate enzymes are surprisingly common in nature, and in some cases arise serendipitously [
112].
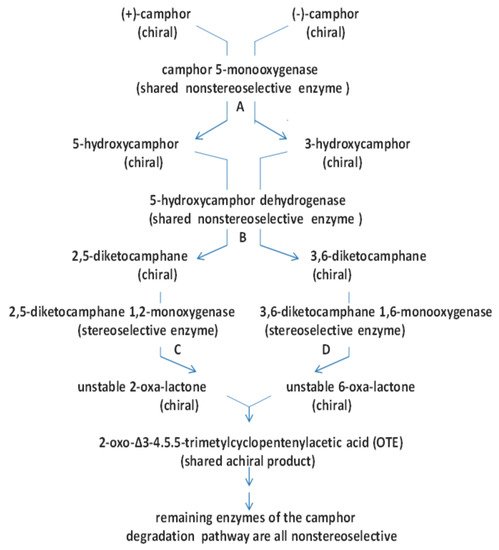
Figure 14. The relationships between nonselective and stereoselective enzymes that constitute the pathway for the degradation of (rac)-camphor to OTE in P. putida ATCC 17453, and the roles of chiral and achiral molecules as substrates and transcriptional regulators. A = induction by (+)-camphor and (−)-camphor, plus product induction by OTE: B = induction by (+)-camphor and (−)-camphor, plus product induction by 2,5-DKC and 3,6-DKC: C = induction by (+)-camphor, (−)-camphor and 2,5-DKC, plus cross-induction by 3,6-DKC, and product induction by OTE: D = ‘from the top’ coordinate induction by (+)-camphor, (−)-camphor, and 3,6-DKC, plus cross-induction by 2,5-DKC, and product induction by OTE: A + B + C + D = ‘from the top’ coordinate induction by (+)-camphor and (−)-camphor.
The most recent contribution to the sporadic six-decade history of the DKCMOs has been the confirmation for the first time that the 533 kb CAM plasmid of
P. putida ATCC 17,453 can function as a totally autonomous extrachromosomal genetic element [
8]. This was a proposal with its origins in Gunsalus’s early quest to characterise the role of oxygen-dependent enzymes in the biogeochemical carbon cycle (vide supra). His interest was fuelled further when his studies in the early 1970s [
74] confirmed that an enzyme suite coded for by the CAM plasmid of
P. putida ATCC 17453, which included four different monooxygenases, was able to catabolise C10 (+)-camphor to three molecules of C2 acetyl-CoA plus one molecule of C4 isobutyryl-CoA. Acetyl-CoA is a key metabolite that is the common end product of a significant number of universal chromosome-coded central intermediary metabolic pathways, and facilitates entry into the CO
2-generating TCA cycle [
113]. Similarly, isobutyryl-CoA is a shared intermediate with the catabolic pathways for pantothenate and valine, both chromosome-coded pathways in pseudomonads [
113]. Irrespective of its metabolic origin, isobutyl-CoA can be further catabolised by chromosome-coded enzymes to the C4 TCA cycle intermediate succinyl-CoA [
114]. Collectively, these activities account for every atom of C10 camphor gaining entry into the CO
2-generating TCA cycle (
Figure 15), thereby supporting Gunsalus’s metabolic autonomy proposal.
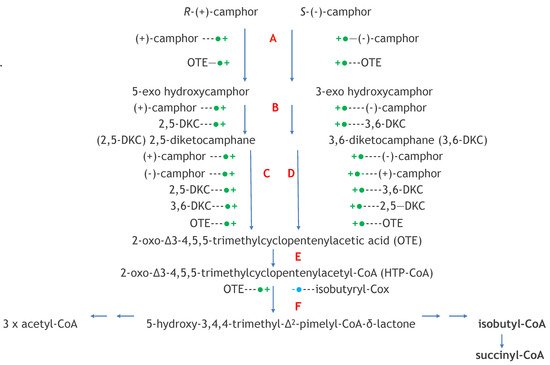
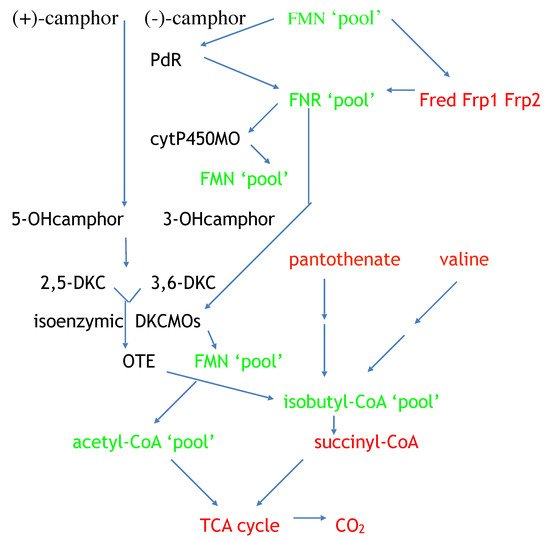
Figure 15. Key interrelationships between CAM plasmid-coded functions and chromosome-coded functions in (rac)-camphor-grown P. putida ATCC 17453. black = biomolecules exclusive to CAM plasmid-directed biochemistry: red = biomolecules exclusive to chromosome-directed biochemistry: green = biomolecules common to both plasmid-directed and chromosome-directed biochemistry. DKC = diketocamphane; FNR = FMNH2.; cytP540MO = camphor 5-monooxygenase; OTE = 2-oxo-△3-4,5,5-trimethylcyclopentylacetic acid; PdR = putidaredoxin.
This piece of Gunsalus gospel then remained unchallenged for almost five decades until 2013 when Iwaki et al. claimed that the chromosome-coded flavin reductase Fred, was the only cognate redox flux partner for the plasmid-coded isoenzymic DKCMOs [
44] which defined a dependence for the total degradation of the carboskeleton of camphor to CO
2 on an identified chromosome-coded function. Iwaki et al.’s claimed exclusive cognate role for Fred was later proven to be incorrect when equivalent roles were confirmed for two other chromosome-coded flavin reductases (Frp1 and Frp2) and, significantly, the plasmid-coded PdR subunit of camphor 5-monooxygenase [
47]. To confirm the functional significance of PdR to CAM plasmid metabolic autonomy, a strategy was developed to investigate whether DKCMO-dependent growth of
P. putida ATCC 17453 on camphor can take place in the absence of functional activities of each of the chromosome-coded FMNH
2-generating enzymes. Advantage was taken of the historical precedent set by the known susceptibility of microbial ferric-flavin reductases inhibition by Zn
2+, a phenomenon first recognised, albeit not fully characterised, nearly 70 years ago [
115,
116,
117,
118]. As a consequence, relevant kinetic studies were used to establish a level of Zn
2+ (30 µM) that completely inhibited Frp1 and Frp2 activity of
P. putida ATCC 17453 but did not otherwise significantly affect growth of the bacterium [
8]. With respect to discounting the activity of Fred, advantage was taken of the established absence of any significant detectible activity of Fred during early and mid-log trophophasic growth of the bacterium on camphor (vide supra). Additionally relevant were extensive prior studies [
42,
47] which confirmed that inoculation of
P. putida ATCC 17453 into a defined medium containing both succinate and (
rac)-camphor results in diauxic growth of the culture. Initial post-inoculation growth of the bacterium occurs exclusively at the expense of succinate, and results in the progressive depletion of succinate to below a key threshold level of 0.5 mM which then triggers a diauxic interlude. During the following 30–40 min, the remaining succinate is then further depleted to below detectible levels, and thereafter induction of the CAM plasmid-coded camphor degradation pathway enzymes is initiated. This then enables the nascent CAM plasmid-coded enzyme activities to initiate a second phase of trophophasic growth of the culture to occur exclusively at the expense of the residual (
rac)-camphor. Significantly, at this stage of diauxic growth, chromosome-coded Fred is undetectable in cells of the bacterium, and only begins to be induced as the culture progresses into idiophasic growth [
47,
70]. By dividing such a diauxic culture into two then adding 30 µM Zn
2+ to only one of the aliquots, and subsequently monitoring both aliquots concurrently throughout the following early and mid log phases of exclusively (
rac)-camphor-dependent growth, it was possible to assess the ability of the Zn
2+-insensitive PdR subunit of camphor 5-monooxygenase to serve as the sole functioning supplier of FMNH
2 to the enantiocomplementary DKCMOs. Notably, the presence of Zn
2+ resulted in no significant difference to either biomass yield, or the activity profiles of the 2,5- and 3,6-DKCMOs, and as expected for trophophasic phase cultures of (
rac)-camphor-grown
P. putida ATCC 17453, no detectible titres of Fred were recorded in any of the tested biomass samples [
8]. Taking the titre of camphor 5-monooxygenase as reflecting that of PdR itself [
94], and assaying Frp1 plus Frp2 as a combined ferric-flavin reductase activity (
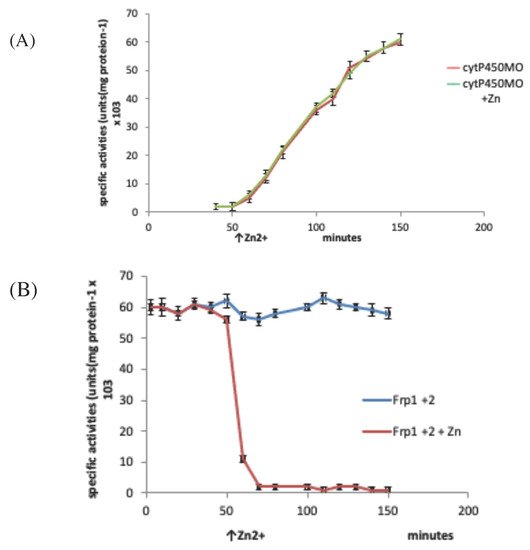
Figure 16), the comparative study conclusively confirmed that the recorded growth of the Zn
2+ supplemented aliquot of the culture was achieved under conditions when the PdR subunit of camphor 5-monooxygenase was the only known functioning FMNH
2-generating activity present in cells of the bacterium. Collectively, these outcomes indicate that, in the absence of active titres for the chromosome-coded enzymes Fred, Frp1, and Frp2, the FMNH
2 necessary for the efficient functioning of the DKCMOs in camphor-grown
P. putida ATCC 17453, which itself is a prerequisite for effective growth, can be supplied by CAM plasmid-coded PdR.
Figure 16. Changes in the activity of (A) camphor 5-monooxygenase (cytP450MO), and (B) the combined Frp1 + Frp2 activity in P. putida ATCC 17453 in the presence and absence of 30 µM Zn2+ added during the diauxic interlude that occurs between the swop from late log growth on succinate to early log growth on (rac)-camphor.
The intriguing question of how PdR is able to simultaneously serve an equivalent auxiliary role for two very different monooxygenase-catalysed steps in the camphor degradation pathway of
P. putida ATCC 17453 (
Figure 17) currently remains unresolved. Interestingly, a study conducted nearly 30 years ago suggested that PdR is a bifunctional enzyme that may be able to act as a NADH-dependent ferrodoxin reductase [
119], while more recently the proven ability of PdR to act as an NAD(H)-dependent dithiol/disulphide oxidoreductase [
120] has substantially strengthened the case for the enzyme being multi-functional. Clearly, the extent of the catalytic versatility of PdR is something that needs to be more thoroughly investigated. The outcomes from this split culture protocol do, however, finally confirm for the first time the proposal made by nearly 50 years ago by Gunsalus [
74] that the CAM plasmid of
P. putida ATCC 17453 functions as a metabolically autonomous unit able to ensure entry of all ten carbon atoms of camphor, a peripheral organic compound, into the chromosome-coded central pathways of metabolism via 3 × C2 acetyl-CoA plus 1 × C4 succinyl-CoA.
Figure 17. Schematic of the two contrasting roles of putidaredoxin reductase (PdR) in camphor-grown P. putida ATCC 17453. (A). cytP450MO (camphor 5-monooxygenase): Pdx (putidaredoxin) (B). 2,5-DKCMO (2,5-diketocamphane 1,2-monooxygenase): DKC (2,5-diketocamphane).
Considered from a different perspective, one possible implication of this and other related previous studies [
47,
70] is that in the absence of membrane-bound subcellular organelles in the prokaryotic cells of camphor-grown
P. putida ATCC 17453, PdR and the various chromosome-coded reductases may, as circumstances dictate, contribute to a ‘pool’ of unbound FMNH
2 (
Figure 14), which is then available as a shared resource to be exploited on an ad hoc basis by the DKCMOs and any other coincidentally present FMNH
2-dependent enzymes. This resonates directly with a speculative suggestion made by Gunsalus nearly 60 years ago [
38], and which has been given additional contemporary support by the very recent confirmed ability of reductase-free biomimetic FMNH
2-generating systems to serve as the redox partner to promote efficient 2,5-DKCMO-catalysed lactonization reactions [
93]. Ironically, this pageant of events provides a paradigm of the six-decade quest to characterise the isoenzymic DKCMOs of camphor-grown
P. putida ATCC 17453. Although not referred to as such because the concept did not exist at the time, Gunsalus’s original rather flippant 1965 suggestion of a ‘pool’ of unbound FMNH
2 effectively delineated the DKCMOs as being what would currently be termed fd-TCMOs. However, while there is no recorded evidence that he made any further reference to his suggested ‘pool’ of unbound FMNH
2, he did within weeks emerge as the leading proponent for the DKCMOs being true flavoproteins containing bound FMN in the active sight [
36]. It was then only after a hiatus of almost half a century that the true status of the isoenzymic DKCMOs as FMNH
2-dependent TCMOs was finally confirmed [
44].
Therefore: even to this day, the episodic history of the isoenzymic DKCMOs induced by the growth of Pseudomonas putida ATCC 17453 on camphor, a plant natural product present in the biosphere, remains an incomplete narrative. This particular journey of scientific discovery commenced with Gunsalus’s quest to resolve the mystery of how the bicyclic terpene can interact with diatomic oxygen to thereby become integrated into the biogeochemical carbon cycle. Now, over 60 years later, it has progressed sufficiently to validate another of Gunsalus’s astute predictions by confirming that the CAM plasmid of P putida ATCC 17453 can function as a totally autonomous metabolic entity. However, the journey is not yet over and the historical epilogue remains incomplete, as illustrated by the current unresolved mystery of how PdR can efficiently serve two distinctly different roles concurrently in the camphor-grown bacterium. As evidenced by this review, the path the journey has taken throughout those intervening six decades has been determined be a veritable potpourri of both positive and negative influences, but that is so often a defining characteristic of the search for the transitional truth of knowledge.