Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Chitosan is one of the most studied natural origin polymers for biomedical applications. Chitosan offers the opportunity to prolong the formulation residence time at mucosal sites; its wound healing properties open possibilities to utilize chitosan in wound dressings with multitargeted activities and more. Chitosan's role in localized antimicrobial therapy is recently gaining increased attention.
- chitosan
- antimicrobial activity
- topical infections
- antimicrobial resistance
- localized therapy
1. Common Skin Infections and Microorganisms
The skin is the largest organ of the human body and is constantly in contact with its surroundings [1], serving as the body’s first defence line [2]. The skin is continuously challenged by essential, opportunistic, and pathogenic microorganisms that could cause infections if the skin barrier is breached or otherwise compromised [3][4]. Even with an effective protective barrier, infections are relatively frequent, becoming a growing concern [5]. The burden of skin infections covers a wide variety of conditions often classified as skin and soft tissue infections (SSTIs). Uncomplicated SSTIs are typically comprised of impetigo, ecthyma, erysipelas, and folliculitis, while complicated SSTIs include more severe, acute wound infections, chronic wound infections, cellulitis, and necrotizing skin infections [6]. U.S. Food and Drug Administration later defined these conditions as acute bacterial skin and skin structure infections [7]. Additionally, chronic wounds are considered a massive burden on the health care systems [8], with Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli among the bacteria most frequently found in these skin infections [9]. Wounds and other skin infections are also polymicrobial in nature, therefore, they are more complex and harder to treat [10].
Superficial fungal infections are an increasing problem affecting between 20–25% of the global population [11][12]. A fungus of importance is Candida albicans, an opportunistic fungus and a natural part of the human microbiota frequently found in microbial communities in chronic wounds [13][14]. Resistance among fungal species is growing, and relatively few treatment options are available [15].
Furthermore, there are many viral infections such as varicella-zoster, herpes zoster, molluscum contagiosum virus, and human herpesvirus 6. However, these infections are often self-limiting and do not require treatment [16]. Herpes simplex virus infections, such as herpes labialis, could be treated locally if the outbreak of the infection is localized in a limited area [17][18]. However, localized, antiviral skin therapy utilizing chitosan delivery systems is underrepresented compared to antibacterial and antifungal treatment.
The burden of infected nonhealing or hard-to-heal wounds, along with other skin diseases, is rapidly increasing, creating a strain on medical services [19]. The term chronic wounds covers a wide variety of conditions, with diabetic foot ulcers, venous leg ulcers, and pressure ulcers being the most relevant [20]. The majority of these wounds are halted in the inflammatory phase, which delays the proliferation and epithelization and decelerates the healing process [21]. Chronic wounds usually are polymicrobial in nature, with bacteria linked to the genetics of each patient [22]. However, S. aureus and P. aeruginosa are recognized as strong contributors to chronicity in nonhealing wounds [23]. Wolcott et al. analyzed bacteria found in chronic wounds of 2963 patients, and S. aureus, Staphylococcus epidermidis, Finegoldia magna, and P. aeruginosa were the most frequently found in these wounds [24]. Yet, the presence of certain microorganisms in the wound bed does not directly indicate an infection [25]. S. aureus and P. aeruginosa are the most common bacteria to produce biofilm networks in the wound bed [21][26], and these biofilms are communities of bacteria living together in extracellular polymeric substances which provide protection and facilitate adhesion to the affected tissue [27]. In addition to the increased protection of microorganisms in biofilms, the matrix allows improved communication between bacteria and increases resistance by allowing bacteria to remain in a dormant state for prolonged periods [21][25]. These biofilm matrices are found in approximately 60–80% of all human infections [28], and bacteria in biofilms are often 1000-fold more resistant than planktonic bacteria, further increasing the challenge in eradicating these pathogens [29]. Fungi are also often a part of the polymicrobial community in chronic wounds, and Candida spp. are regularly reported as the most recurrent fungi [13]. Wounds or other skin breaches could potentially cause other forms of SSTIs if left untreated [30]. Utilizing topical antibiotics in the treatment of chronic wounds is not uncommon; however, the evidence behind their use is somewhat limited [31]. The selection of the type of antibiotic is dependent on the microbial picture [23][28].
2. Challenges of Antimicrobial Treatment and Delivery to the Skin
The skin represents an attractive route in the therapy of localized microbial infections, mainly due to the potential of higher local drug concentrations [32]. Additionally, the demand for therapeutic options for skin infection is increasing with growing numbers of SSTIs globally [33]. However, several limitations are linked to the skin as a target site for delivering antimicrobial compounds.
The skin structure comprises three main layers; epidermis, dermis, and hypodermis [1]. The epidermis mainly consists of keratinocytes and is where the binding of pharmaceutical compounds, metabolism, and active transport occurs [34]. Below the epidermis is a layer of connective tissue composed of fibroblasts, namely the dermis, responsible for the structure and elasticity of the skin [35]. The hypodermis, or the subcutaneous tissue, is the innermost layer of the skin and consists of connective tissue, however, looser than the connective tissue in the dermis. The main functions of the hypodermis are protection from physical impact, temperature regulation, and energy storage [35]. The uppermost and primary protective layer, the stratum corneum (SC), plays a key role in dermal delivery [36]. The SC limits the penetration of active compounds due to the lipophilic nature of the barrier, which prevents absorption of molecules with an molecular weight (MW) of more than 500 Da [36][37][38]. The SC is structured in a distinct way, often referred to as the brick-and-mortar model, where the corneocytes represent the bricks and the lipid matrix represents the mortar [1]. In the localized treatment of dermal infections, the aim is to assure penetration into the infection site while avoiding the potential absorption. The penetration of the active compound might be slow, creating challenges in reaching the desired concentration [39]. Determining the concentration of the compound within the skin layers, as well as determining the compound clearance and degradation from these layers, is challenging as well as [40].
In localized skin infections, the heterogeneity between patients might pose another challenge. The skin condition might be altered if the patient suffers from SSTIs and skin impairments in general. In the case of wounds or impaired skin, the skin’s barrier function might be lost, and penetration or permeation through the skin might increase [1]. This might lead to lowered concentrations of the antimicrobial compounds in the intended site and could increase the potential of systemic side effects. Furthermore, the skin or wound environment might be altered, and the skin barrier weakened because of, and depending on, the given condition, e.g., infection, wounds, lesions, or inflammation, or thickened, due to ichthyosis or cancer. In these cases, the absorption into or through the skin is different from normal or healthy skin [41]. Additionally, impairment of the skin, especially if the damage reaches into the deeper skin layers or is bleeding, could cause alteration to the pH on the surface from the naturally more acidic milieu on the SC. This more neutral environment is beneficial for the growth of many pathogenic microorganisms, further increasing the challenge in treating the SSTIs [42]. Impaired skin, especially wounds and lesions, could contain high volumes of exudate, essential for maintaining a proper healing cascade and maintaining a moist wound bed. However, extensive volumes of exudate could lead to further reduced healing [43][44] and introduce challenges in maintaining contact between therapeutic formulation and the infection site, leading to lower retention or residence time of the therapeutic in the affected area [25]. These and other factors, such as low permeation or degradation, could result in the need for frequent drug administration onto a damaged and painful area [45] and further impaired healing [45]. In addition, the potential of local side effects or skin irritation needs to be accounted for [32]. Allergic contact dermatitis is not uncommon [36]. Occlusion effects could also potentially increase pH and temperature at the treatment site and cause skin irritation; however, these effects are often easy to avoid [36].
The most significant challenges in antimicrobial skin therapy are the numerous variations in conditions and the microbial diversity between patients and within the same SSTI [1][31]. The microbial picture depends on the patients’ genetics and the environment and varies between different sites on the human body [22][46]. Furthermore, biofilms can be found in up to 80% of all infections [28]. Antimicrobial resistance results from different mechanisms, such as restricted penetration of antimicrobial compounds into the biofilm matrix, altered metabolic activity in the microorganisms, altered gene expression, and microorganisms that remain in a dormant state. Topical therapy might increase the local concentration of antimicrobial compounds, such as mupirocin, metronidazole, and silver sulfadiazine [47]. Moreover, the biofilms are often firmly adhering to skin structures and might stretch further into the deeper skin layers, rendering removal challenging [25][43].
3. Tackling the Challenges of Infected Skin—The Delivery Strategies, Systems, and Scaffolds
In SSTIs and wound healing, chitosan is utilized to produce various drug delivery systems and scaffolds for wound healing and tissue regeneration. The range of these systems and scaffolds varies between polymer-based gels, nanofibrous scaffolds, and particles for regeneration, antimicrobial activity, and carrying the antimicrobial compounds. However, most systems and scaffolds are intended to heal wounds [48].
The scaffold should protect the wound from external contamination, maintain moisture, allow gas exchange, prevent bacterial growth, or eradicate bacteria in the wound bed, facilitate all stages of the wound healing cascade, and it should be biodegradable and biocompatible [49][48][50]. Chitosan possesses many of these necessary properties [51][52]. Additionally, it can assist in a sustained and controlled release of pharmaceutical compounds, such as antimicrobials, both in micro and nanotechnology [50][53][54]. Its retention in the skin could be improved while maintaining the balance between retention and penetration to ensure sufficient concentrations of the pharmaceutical compound [55]. Furthermore, advanced scaffolds could mimic the extracellular matrix to further promote skin healing [50]. With its intrinsic antimicrobial properties, chitosan is an ideal material in drug delivery systems and scaffolds intended for localized therapy.
These delivery systems could be classified according to composition and size range. In skin repair and wound healing, these could be classified as nanoparticles, nanocomposites, coatings, and scaffolds, depending on their intended use and attribution in the therapy [49]. As a bioactive polymer, chitosan is easily developed for gels, membranes, nanofibers, microparticles, nanoparticles, sponges, and scaffolds [56]. Chitosan-based delivery systems targeting skin infections are summarized in Figure 1.
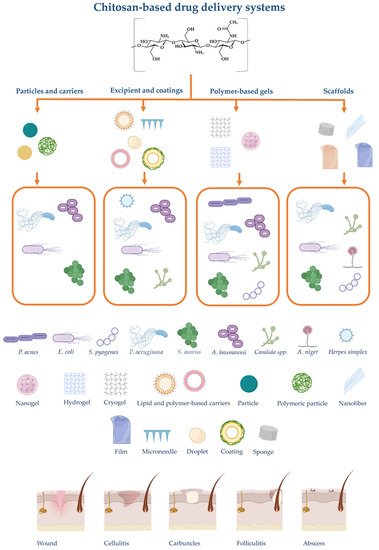
Figure 1. Summary of chitosan-based drug delivery systems and scaffolds intended for skin administration with their respective targeted microorganisms and examples of skin infections. The illustration is created with BioRender.com.
This entry is adapted from the peer-reviewed paper 10.3390/md19120697
References
- Salatin, Sara; Lotfipour, Farzaneh; Jelvehgari, Mitra; A brief overview on nano-sized materials used in the topical treatment of skin and soft tissue bacterial infections.. Expert Opinion on Drug Delivery 2019, 16, 1313-1331, 10.1080/17425247.2020.1693998.
- Tavakoli, Shima; Klar, Agnes S.; Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169, 10.3390/biom10081169.
- Byrd, Allyson L.; Belkaid, Yasmine; Segre, Julia A.; The human skin microbiome. Nature Reviews Genetics 2018, 16, 143-155, 10.1038/nrmicro.2017.157.
- Lei, Vivian; Petty, Amy J.; Atwater, Amber R.; Wolfe, Sarah A.; MacLeod, Amanda S.; Skin Viral Infections: Host Antiviral Innate Immunity and Viral Immune Evasion. Frontiers in Immunology 2020, 11, 593901, 10.3389/fimmu.2020.593901.
- O'Dell, Michael L.; Skin and Wound Infections: An Overview. American Family Physician 1998, 57, 2424-2432, .
- Esposito, Silvano; Noviello, Silvana; Leone, Sebastiano; Epidemiology and microbiology of skin and soft tissue infections. Current Opinion in Infectious Diseases 2016, 29, 109-115, 10.1097/qco.0000000000000239.
- Leong, Hoe Nam; Kurup, Asok; Tan, Mak Yong; Kwa, Andrea Lay Hoon; Liau, Kui Hin; Wilcox, Mark H.; Management of complicated skin and soft tissue infections with a special focus on the role of newer antibiotics. Infection and Drug Resistance 2018, 11, 1959-1974, 10.2147/idr.s172366.
- Maheswary, Thambirajoo; Nurul, Asma A.; Fauzi, Mh B.; The Insights of Microbes’ Roles in Wound Healing: A Comprehensive Review. Pharmaceutics 2021, 13, 981, 10.3390/pharmaceutics13070981.
- Negut, Irina; Grumezescu, Valentina; Grumezescu, Alexandru Mihai; Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392, 10.3390/molecules23092392.
- Tomic-Canic, Marjana; Burgess, Jamie L.; O’Neill, Katelyn E.; Strbo, Natasa; Pastar, Irena; Skin Microbiota and its Interplay with Wound Healing. American Journal of Clinical Dermatology 2020, 21, 36-43, 10.1007/s40257-020-00536-w.
- Ameen, Mahreen; Epidemiology of superficial fungal infections. Clinics in Dermatology 2010, 28, 197-201, 10.1016/j.clindermatol.2009.12.005.
- James, Spencer L.; Abate, Degu; Abate, Kalkidan Hassen; Abay, Solomon M.; Abbafati, Cristiana; Abbasi, Nooshin; Abbastabar, Hedayat; Abd-Allah, Foad; Abdela, Jemal; Abdelalim, Ahmed; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018, 392, 1789-1858, 10.1016/s0140-6736(18)32279-7.
- Dowd, S. E.; Delton Hanson, J.; Rees, E.; Wolcott, R. D.; Zischau, A. M.; Sun, Y.; White, J.; Smith, D. M.; Kennedy, J.; Jones, C. E.; et al. Survey of fungi and yeast in polymicrobial infections in chronic wounds. Journal of Wound Care 2011, 20, 40-47, 10.12968/jowc.2011.20.1.40.
- Spampinato, Claudia; Leonardi, Darío; Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. BioMed Research International 2013, 2013, 204237, 10.1155/2013/204237.
- Gupta, Aditya K.; Venkataraman, Maanasa; Renaud, Helen J.; Summerbell, Richard; Shear, Neil H.; Piguet, Vincent; The increasing problem of treatment‐resistant fungal infections: a call for antifungal stewardship programs. International Journal of Dermatology 2021, 60, e474-e479, 10.1111/ijd.15495.
- Thandi, Charankumal Singh; Whittam, Lindsay; Diagnosis and management of common viral skin infections. Prescriber 2021, 32, 10-14, 10.1002/psb.1907.
- Donalisio, Manuela; Leone, Federica; Civra, Andrea; Spagnolo, Rita; Ozer, Ozgen; Lembo, David; Cavalli, Roberta; Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46, 10.3390/pharmaceutics10020046.
- Park, Kyoung C.; Han, Won S.; Viral Skin Infections. Drugs 2002, 62, 479-490, .
- Martinengo, Laura; Olsson, Maja; Bajpai, Ram; Soljak, Michael; Upton, Zee; Schmidtchen, Artur; Car, Josip; Järbrink, Krister; Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Annals of Epidemiology 2019, 29, 8-15, 10.1016/j.annepidem.2018.10.005.
- Zhao, Ruilong; Liang, Helena; Clarke, Elizabeth; Jackson, Christopher; Xue, Meilang; Inflammation in Chronic Wounds. International Journal of Molecular Sciences 2016, 17, 2085, 10.3390/ijms17122085.
- Gajula, Bhargav; Munnamgi, Sinduja; Basu, Somprakas; How bacterial biofilms affect chronic wound healing: a narrative review. International Journal of Surgery: Global Health 2020, 3, e16, 10.1097/gh9.0000000000000016.
- Tipton, Craig D.; Wolcott, Randall D.; Sanford, Nicholas E.; Miller, Clint; Pathak, Gita; Silzer, Talisa K.; Sun, Jie; Fleming, Derek; Rumbaugh, Kendra P.; Little, Todd D.; et al. Patient genetics is linked to chronic wound microbiome composition and healing. PLOS Pathogens 2020, 16, e1008511, 10.1371/journal.ppat.1008511.
- Serra, Raffaele; Grande, Raffaele; Butrico, Lucia; Rossi, Alessio; Settimio, Ugo Francesco; Caroleo, Benedetto; Amato, Bruno; Gallelli, Luca; de Franciscis, Stefano; Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Review of Anti-infective Therapy 2015, 13, 605-613, 10.1586/14787210.2015.1023291.
- Wolcott, Randall D.; Hanson, John D.; Rees, Eric J.; Koenig, Lawrence D.; Phillips, Caleb D.; Wolcott, Richard A.; Cox, Stephen B.; White, Jennifer S.; Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair and Regeneration 2016, 24, 163-174, 10.1111/wrr.12370.
- Kaiser, Pia; Wächter, Jana; Windbergs, Maike; Therapy of infected wounds: overcoming clinical challenges by advanced drug delivery systems. Drug Delivery and Translational Research 2021, 11, 1545-1567, 10.1007/s13346-021-00932-7.
- Drago, Francesco; Gariazzo, Lodovica; Cioni, Margherita; Trave, Ilaria; Parodi, Aurora; The microbiome and its relevance in complex wounds. European Journal of Dermatology 2019, 29, 6-13, 10.1684/ejd.2018.3486.
- Wu, Yuan-Kun; Cheng, Nai-Chen; Cheng, Chao-Min; Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends in Biotechnology 2019, 37, 505-517, 10.1016/j.tibtech.2018.10.011.
- Omar, Amin; Wright, J. B.; Schultz, Gregory; Burrell, Robert; Nadworny, Patricia; Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9, 10.3390/microorganisms5010009.
- Yue, Lin; Wang, Min; Khan, Imran Mahmood; Xu, Jianguo; Peng, Chifang; Wang, Zhouping; Preparation, characterization, and antibiofilm activity of cinnamic acid conjugated hydroxypropyl chitosan derivatives. International Journal of Biological Macromolecules 2021, 189, 657-667, 10.1016/j.ijbiomac.2021.08.164.
- Raff, Adam B.; Kroshinsky, Daniela; Cellulitis: A Review. JAMA 2016, 316, 325-337, 10.1001/jama.2016.8825.
- Williamson, Deborah A.; Carter, Glen P.; Howden, Benjamin P.; Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clinical Microbiology Reviews 2017, 30, 827-860, 10.1128/cmr.00112-16.
- Lam, P. L.; Lee, K. K. H.; Wong, R. S. M.; Cheng, G. Y. M.; Bian, Z. X.; Chui, C. H.; Gambari, R.; Recent advances on topical antimicrobials for skin and soft tissue infections and their safety concerns. Critical Reviews in Microbiology 2018, 44, 40-78, 10.1080/1040841x.2017.1313811.
- Martinez, Nicole; Skin and Soft-Tissue Infections: It's More Than Just Skin Deep. Advanced Emergency Nursing Journal 2020, 42, 196-203, 10.1097/tme.0000000000000312.
- Cui, Mingyue; Wiraja, Christian; Chew, Sharon Wan Ting; Xu, Chenjie; Nanodelivery Systems for Topical Management of Skin Disorders. Molecular Pharmaceutics 2021, 18, 491-505, 10.1021/acs.molpharmaceut.0c00154.
- Vitorino, Carla; Sousa, Joao; Pais, Alberto; Overcoming the Skin Permeation Barrier: Challenges and Opportunities. Current Pharmaceutical Design 2015, 21, 2698-2712, 10.2174/1381612821666150428124053.
- Iqbal, Babar; Ali, Javed Baboota, Sanjula; Recent advances and development in epidermal and dermal drug deposition enhancement technology. International Journal of Dermatology 2018, 57, 646-660, 10.1111/ijd.13902.
- Antimisiaris, S. G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S.; Overcoming barriers by local drug delivery with liposomes. Advanced Drug Delivery Reviews 2021, 174, 53-86, 10.1016/j.addr.2021.01.019.
- Badilli, Ulya; Gumustas, Mehmet; Uslu, Bengi; Ozkan, Sibel A.. Chapter 9 - Lipid-based nanoparticles for dermal drug delivery; Grumezescu, Alexandru Mihai, Eds.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 369-413.
- Zoabi, Amani; Touitou, Elka; Margulis, Katherine; Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids and Interfaces 2021, 5, 18, 10.3390/colloids5010018.
- Maciel Tabosa, Maria Alice; Hoppel, Magdalena; Bunge, Annette L.; Guy, Richard H.; Delgado-Charro, M. Begoña; Predicting topical drug clearance from the skin. Drug Delivery and Translational Research 2020, 11, 729-740, 10.1007/s13346-020-00864-8.
- Alnasif, Nesrin; Zoschke, Christian; Fleige, Emanuel; Brodwolf, Robert; Boreham, Alexander; Rühl, Eckart; Eckl, Katja-Martina; Merk, Hans-Friedrich; Hennies, Hans Christian; Alexiev, Ulrike; et al. Penetration of normal, damaged and diseased skin — An in vitro study on dendritic core–multishell nanotransporters. Journal of Controlled Release 2014, 185, 45-50, 10.1016/j.jconrel.2014.04.006.
- Wallace, Laura A.; Gwynne, Lauren; Jenkins, Tobias; Challenges and opportunities of pH in chronic wounds. Therapeutic Delivery 2019, 10, 719-735, 10.4155/tde-2019-0066.
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N.; Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56, 10.3390/antibiotics9020056.
- Sweeney, India R.; Miraftab, Mohsen; Collyer, Graham; A critical review of modern and emerging absorbent dressings used to treat exuding wounds. International Wound Journal 2012, 9, 601-612, 10.1111/j.1742-481x.2011.00923.x.
- Lipsky, Benjamin A.; Hoey, Christopher; Topical Antimicrobial Therapy for Treating Chronic Wounds. Clinical Infectious Diseases 2009, 49, 1541-1549, 10.1086/644732.
- Grice, Elizabeth A.; Segre, Julia A.; The skin microbiome. Nature Reviews Genetics 2011, 9, 244-253, 10.1038/nrmicro2537.
- Ciofu, Oana; Rojo-Molinero, Estrella; Macià, María D.; Oliver, Antonio; Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304-319, 10.1111/apm.12673.
- Negut, Irina; Dorcioman, Gabriela; Grumezescu, Valentina; Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010, 10.3390/polym12092010.
- Mihai, Mara Madalina; Dima, Monica Beatrice; Dima, Bogdan; Holban, Alina Maria; Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176, 10.3390/ma12132176.
- Saghazadeh, Saghi; Rinoldi, Chiara; Schot, Maik; Kashaf, Sara Saheb; Sharifi, Fatemeh; Jalilian, Elmira; Nuutila, Kristo; Giatsidis, Giorgio; Mostafalu, Pooria; Derakhshandeh, Hossein; et al. Drug delivery systems and materials for wound healing applications. Advanced Drug Delivery Reviews 2018, 127, 138-166, 10.1016/j.addr.2018.04.008.
- Okur, Mehmet Evren; Karantas, Ioannis D.; Şenyiğit, Zeynep; Üstündağ Okur, Neslihan; Siafaka, Panoraia I.; Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian Journal of Pharmaceutical Sciences 2020, 15, 661-684, 10.1016/j.ajps.2019.11.008.
- Matica, Mariana Adina; Aachmann, Finn Lillelund; Tøndervik, Anne; Sletta, Håvard; Ostafe, Vasile; Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. International Journal of Molecular Sciences 2019, 20, 5889, 10.3390/ijms20235889.
- Basnet, Purusotam; Škalko-Basnet, Nataša; Nanodelivery systems for improved topical antimicrobial therapy. Current Pharmaceutical Design 2013, 19, 7237-7243, .
- Dos Santos Ramos, Matheus Aparecido; Da Silva, Patrícia Bento; Spósito, Larissa; De Toledo, Luciani Gaspar; Bonifácio, Bruna Vidal; Rodero, Camila Fernanda; Dos Santos, Karen Cristina; Chorilli, Marlus; Bauab, Taís Maria; Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. International Journal of Nanomedicine 2018, 13, 1179-1213, 10.2147/ijn.s146195.
- Vogt, Annika; Wischke, Christian; Neffe, Axel T.; Ma, Nan; Alexiev, Ulrike; Lendlein, Andreas; Nanocarriers for drug delivery into and through the skin — Do existing technologies match clinical challenges?. Journal of Controlled Release 2016, 242, 3-15, 10.1016/j.jconrel.2016.07.027.
- Ahmed, Shakeel; Ikram, Saiqa; Chitosan Based Scaffolds and Their Applications in Wound Healing. Achievements in the Life Sciences 2016, 10, 27-37, 10.1016/j.als.2016.04.001.
This entry is offline, you can click here to edit this entry!
