meta-Tyrosine (m-Tyr) is a non-proteinogenic isomer of p-tyrosine (Tyr) and is an antimetabolite of proteinogenic amino acid phenylalanine (Phe). This compound can be found in animal and plant cells.
- allelochemical
- non-proteinogenic amino acid
- fescue
- phenylalanine antimetabolite
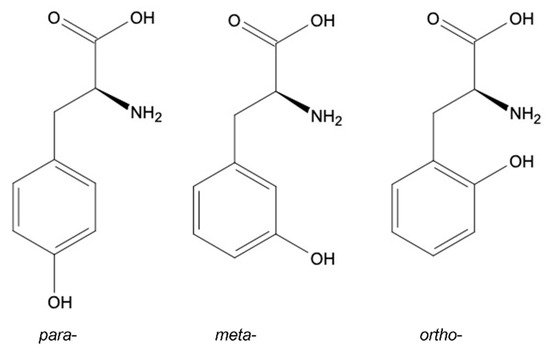
Tyrosine Structural Isomers: meta-, ortho-, para-Tyr
Fescue as the Biological Source of m-Tyr
The direct mode of action linked to m-Tyr toxicity is its incorporation into the proteins[9]. In Escherichia coli incorporation occurs through the binding of m-Tyr to the tRNAPhe [10]. As was shown for bacteria and human cells the cytoplasmic or mitochondrial aminoacyl-tRNA synthetases are prone to catalyzing the binding of tRNAPhe with m-Tyr[11], thus Tyr isomers at higher concentration compete with Phe, for tRNAPhe [12]. The incorporation of m-Tyr into plant proteins was also demonstrated for 5-days old Arabidopsis seedlings [13].
The toxicity of m-Tyr might be overcome by the Phe application[14]. The mechanism of recovery effect is most probably based on the competition between Phe and m-Tyr[15]. m-Tyr toxicity is also linked to altered reactive oxygen species (ROS) metabolism including accumulation of carbonylated proteins[14][16]. Besides alteration of ROS metabolism, m-Tyr has an impact on reactive nitrogen species (RNS) content [16][17] .
In humans, elevated concentration of this Tyr isomer occurs in neurodegenerative diseases and diseases associated with oxidative stress and/or ageing: diabetes, artherosclerosis and others[18]. Moreover, m-Tyr can play a significant role in the cancer cells in animals. The concomitant tumor resistance is the phenomenon of inhibition of secondary tumor implants or metastasis development in hosts, that already are affected by the primary tumor[19]. m-Tyr and o-Tyr were found to be a factor leading to that resistance as they were discovered in the serum of tumor-bearing mice (Mus musculus). Administration of these NPAAs inhibited the growth of tumors in the murine models of cancer[20]. As was discussed, while secondary tumors are inhibited by circulating m-Tyr, the primary tumor microenvironment is protected by an accumulation of AA with counteracting properties (i.e. Phe)[21]. The primary tumor affected ROS generation resulting in the increased m-Tyr and o-Tyr content[20]. This effect was observed in different human tumors type (prostate tumor, lung anaplastic, and nasopharyngeal carcinoma), where the application of m-Tyr led to inhibition of cancer proliferation[22]. The treatment of cancer cells with m-Tyr induced the autophagy, however, the application of Phe reversed the toxic effect of m-Tyr on secondary cancer growth[20][22]. Studies on the key role of non-proteinogenic Tyr isomers in the mechanism of concomitant tumor resistance have shown an antiproliferative and anti-metastaic effect of m-Tyr[20][21][22]. Moreover, m-Tyr can stop the growth of cells from tumor fragments that could have remained post surgery.
Phe might overcome the toxic effect of m-Tyr. Increased Phe level may be achieved by the inhibition of the activity of Phe hydroxylase (the enzyme responsible for Phe catabolism). On the other hand, the degradation of Tyr isomers may occur by the higher activity of tyrosine aminotransferase (Tyr-AT) – first enzyme in the Tyr catabolism pathway[18].
This entry is adapted from the peer-reviewed paper 10.3390/plants10122800
References
- Vranova, V.; Rejsek K.; Skene K.R.; Formanek P.; Non-protein amino acids: plant, soil and ecosystem interactions. Plant and Soil 2010, 342, 31-48, 10.1007/s11104-010-0673-y.
- Ipson B.R.; Fisher A.L.; Roles of the tyrosine isomers meta- tyrosine and ortho- tyrosine in oxidative stress. Ageing Research Reviews 2016, 27, 93-107, 10.1016/j.arr.2016.03.005.
- Molnár A.G.; Kun S.; Sélley E.; Kertész M.; Szélig L.; Csontos C.; Böddi K.; Bogár L.; Miseta A.; Role of Tyrosine Isomers in Acute and Chronic Diseases Leading to Oxidative Stress - A Review. Current Medicinal Chemistry 2016, 23, 667-685, 10.2174/0929867323666160119094516.
- Ruemmele B.A.; Wipff J.; Brilman L.; Hignight K.; Fine-Leaved Fescue Species. In Turfgrass Biology, Genetics and Breeding; Casler M.D.; Duncan R.R. Eds.; JohnWiley & Sons: New York, NY, USA, 2003; pp. 129–174.
- Bertin C.; Paul R.N.; Duke S.O.; Weston L.A.; Laboratory assessment of the allelopathic effects of fine leaf fescues.. Journal of Chemical Ecology 2003, 29, 1919-1937, 10.1023/a:1024810630275.
- Bertin C.; Weston L.A.; Huang T; Jander G.; Owens T.; Meinwald J.; Schroeder F. C.; Grass roots chemistry: meta-Tyrosine, an herbicidal nonprotein amino acid. Proceedings of the National Academy of Sciences 2007, 104, 16964-16969, 10.1073/pnas.0707198104.
- Duke S. O.; The emergence of grass root chemical ecology. Proceedings of the National Academy of Sciences 2007, 104, 16729-16730, 10.1073/pnas.0707837104.
- Bertin C.; Harmon R; Akaogi M; Weidenhamer J.D.; Weston L.A.; Assessment of the Phytotoxic Potential of m-Tyrosine in Laboratory Soil Bioassays. Journal of Chemical Ecology 2009, 35, 1288-1294, 10.1007/s10886-009-9707-4.
- Zhang W.; Ames B.D.; Walsh C.T.; Identification of Phenylalanine 3-Hydroxylase for meta-Tyrosine Biosynthesis. Biochemistry 2011, 50, 5401-5403, 10.1021/bi200733c.
- Huang T.; Rehak L.; Jander G.; meta-Tyrosine in Festuca rubra ssp. commutata (Chewings fescue) is synthesized by hydroxylation of phenylalanine. Phytochemistry 2012, 75, 60-66, 10.1016/j.phytochem.2011.09.018.
- Gurer-Orhan H.; Ercal N.; Mare S.; Pennathur S.; Orhan H.; Heinecke J.W.; Misincorporation of free m-tyrosine into cellular proteins: a potential cytotoxic mechanism for oxidized amino acids. Biochemical Journal 2006, 395, 277-284, 10.1042/bj20051964.
- Bullwinkle T.J.; Reynolds N.M.; Raina M.; Moghal A.; Matsa E.; Rajkovic A.; Kayadibi H.; Fazlollahi F.; Ryan C.; Howitz N.; et al. Oxidation of cellular amino acid pools leads to cytotoxic mistranslation of the genetic code. eLife 2014, 3, e02501, 10.7554/elife.02501.
- Klipcan L.; Moor N.; Kessler N.; Safro M.G.; Eukaryotic cytosolic and mitochondrial phenylalanyl-tRNA synthetases catalyze the charging of tRNA with the meta-tyrosine. Proceedings of the National Academy of Sciences 2009, 106, 11045-11048, 10.1073/pnas.0905212106.
- Rodgers K.J.; Samardzic K.; Main B.J.; Toxic Nonprotein Amino Acids. In Plant Toxins. Toxicology; Gopalakrishnakone P.; Regina Carlini C.; Ligabue-Braun R.; Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 263–285.
- Peters E. J.; Mohammed Zam A. H. B.; Allelopathic Effects of Tall Fescue Genotypes. Agronomy Journal 1981, 73, 56-58, 10.2134/agronj1981.00021962007300010013x.
- Andrzejczak O.; Krasuska U.; Olechowicz J.; Staszek P.; Ciacka K.; Bogatek R.; Hebelstrup K.; Gniazdowska A.; Destabilization of ROS metabolism in tomato roots as a phytotoxic effect of meta -tyrosine. Plant Physiology and Biochemistry 2018, 123, 369-377, 10.1016/j.plaphy.2017.12.024.
- Rosenthal G. A.; l -Canavanine: a higher plant insecticidal allelochemical. Amino Acids 2001, 21, 319-330, 10.1007/s007260170017.
- Widhalm J.R.; Gutensohn M.; Yoo H.; Adebesin F.; Qian Y.; Guo L.; Jaini R.; Lynch J.H.; McCoy R.M.; Shreve J.T.; et al. Identification of a plastidial phenylalanine exporter that influences flux distribution through the phenylalanine biosynthetic network. Nature Communications 2015, 6, 8142, 10.1038/ncomms9142.
- Howitz N.; Su T.; Lazazzera B.A.; Meta-Tyrosine Induces Cytotoxic Misregulation of Metabolism in Escherichia coli. Journal of Molecular Biology 2020, 432, 166716, 10.1016/j.jmb.2020.11.015.
- Chiarella P.; Bruzzo J; Meiss R.P.; Ruggiero R.A.; Concomitant tumor resistance. Cancer Letters 2012, 324, 133-141, 10.1016/j.canlet.2012.05.021.
- Raúl A. Ruggiero; Juan Bruzzo; Paula Chiarella; Pedro Di Gianni; Martín A. Isturiz; Susana Linskens; Norma Speziale; Roberto P. Meiss; Oscar D. Bustuoabad; Christiane D. Pasqualini; et al. Tyrosine Isomers Mediate the Classical Phenomenon of Concomitant Tumor Resistance. Cancer Research 2011, 71, 7113-7124, 10.1158/0008-5472.can-11-0581.
- Ruggiero R.A.; Bruzzo J.; Chiarella P.; Bustuoabad O.D.; Meiss R.P.; Pasqualini C.D.; Concomitant Tumor Resistance: The Role of Tyrosine Isomers in the Mechanisms of Metastases Control: Figure 1.. Cancer Research 2012, 72, 1043-1050, 10.1158/0008-5472.can-11-2964.
