Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Mitochondria are vital organelles in eukaryotic cells that control diverse physiological processes related to energy production, calcium homeostasis, the generation of reactive oxygen species, and cell death. Several studies have demonstrated that structural and functional mitochondrial disturbances are involved in the development of different neuroinflammatory (NI) and neurodegenerative (ND) diseases (NI&NDDs) such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis.
- mitochondria
- neuroinflammatory diseases
- neurodegenerative diseases
1. Introduction
Mitochondria are vital organelles in eukaryotic cells that control diverse physiologi-cal processes related to the production of energy and also cellular processes such as cell death, calcium homeostasis, and the generation and modulation of reactive oxygen spe-cies (ROS) levels [1][2][3]. Mitochondria are highly dynamic and regulated by a fine balance between biogenesis and the degradation of defective organelles [4][5]. The shape, distribution, and size of mitochondria are controlled by coordinated cycles of fission and fusion known as mitochondrial dynamics [6], whereas damaged mitochondria are selectively removed by mitophagy. Biogenesis, mitochondrial dynamics, and clearance are crucial for the functional state of mitochondria. Abnormalities or an imbalance affecting these events may have detrimental effects on mitochondria biology and cell viability [7][8]. Diverse studies performed in cell cultures, animal models and patients have shown that disturbances in mitochondrial structure and function are involved in neurodegeneration leading to motor and cognitive deficits in neuroinflammatory (NI) and neurodegenerative (ND) diseases (NI&NDDs) such as multiple sclerosis (MS), Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). Indeed, a common hallmark of different NI&NDDs is a bioenergetic deficit resulting from mitochondrial dysfunction. Furthermore, the impaired function of mitochondria increases ROS production and oxidative stress exacerbating mitochondrial damage and the progression of neurodegeneration [9][10]. In addition, structural and functional altera-tions in mitochondria are also associated with the pathological accumulation of protein aggregates in NI&NDDs. Remarkably, the restoration of mitochondrial function leads to cell damage recovery and the amelioration of clinical symptoms in cellular and animal models of NI&NDDs [11][12][13][14][15][16]. Therefore, strategies designed to restore mitochondrial homeostasis represent potential therapies for NI&NDDs and should consider not only physicochemical characteristics of drugs but also delivery formulations and biological barriers in order to reach intracellular targets in the central nervous system (CNS) and to mitigate systemic side effects.
2. Organization of Mitochondria
Mitochondria consist of a double membrane with an intermembrane space and an internal mitochondrial matrix (MM) that contains the mitochondrial DNA (mtDNA). The outer mitochondrial membrane (OMM) contains the voltage-dependent anion channel (VDAC) and the permeability transition pore (mPTP) associated with the unspecific trans-location of small molecules (1-5 kDa) through passive diffusion [17][18]. The inner mito-chondrial membrane (IMM) is organized in folds or cristae and contains the electron transport chain (ETC) where the oxidative phosphorylation (OXPHOS) of adenosine di-phosphate (ADP) proceeds (Figure 1). The mtDNA, located in the MM, encodes the protein subunits of respiratory chain complexes I, III, IV, and V, along with RNA components for mitochondrial protein synthesis [19]. Complex II is encoded entirely by autosomal genes [20]. These ETC complexes catalyze redox reactions from reduced dinucleotide do-nors to molecular O2, generating a mitochondrial membrane potential (MMP) along the IMM by pumping protons from the MM to the intermembrane space. Finally, the return of protons into the MM through an ATP synthase present in IMM drives ATP synthesis from ADP and inorganic phosphate [21][22].
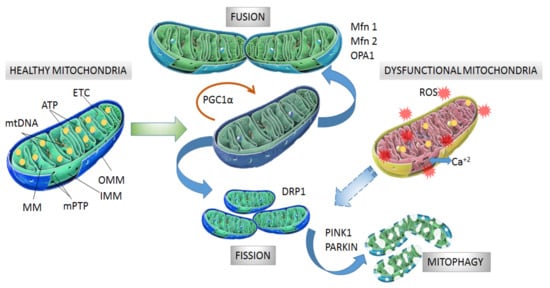
Figure 1. Structure and processes involved in dynamics of healthy and dysfunctional mitochondria. Healthy mitochondria display coordinated and dynamic processes of fusion and fission in order to regulate their morphology, size, and number. After mitochondrial biogenesis guided by PGC-1α protein, fusion generates an interconnected mitochondrial network, which is orchestrated by OPA1, Mfn1 and Mfn2 proteins. Fission results in small size mitochondria without mtDNA replication due to fragmentation and separation from the mitochondrial network, which is a process driven by dynamin- related protein (DRP1). Fragmented mitochondria are degraded by mitophagy, which is a process involving PINK1 and PARKIN proteins. Dysfunctional mitochondria showing alterations in structure and function in neurodegeneration are degraded by mitophagy. Mitochondrial dynamics are maintained by constant activity and precise balance between the biogenesis and clearance of fragmented and defective organelles. mtDNA: mitochondrial DNA, ATP: adenosine triphosphate, ETC: electron transport chain, MM: mitochondrial matrix, mPTP: permeability transition pore, OMM: outer mitochondrial membrane, IMM: inner mitochondrial membrane, PGC-1α: peroxisome proliferator activated receptor-gamma coactivator 1-alpha, Mfn1 and Mfn2: mitofusins 1 and 2, OPA1: optical atrophy 1 protein, DRP1: dynamin related protein, PINK1: PTEN-induced kinase 1, PARKIN: Parkin RBR E3 ubiquitin-protein ligase.
Since ETC operates under the presence of O2, the process involves one-electron redox reactions, which demonstrates that mitochondria are the major intracellular source of ROS under normal physiological conditions [23][24][25][26]. Indeed, mitochondria generate almost 90% of cellular ROS [3]. Under pathological conditions, altered ETC function causes an exacerbation of mitochondrial ROS production leading to bioenergetic impairment and cellular and tissue damage by oxidative stress; contributing to a progressive ND process [27][28].
A relevant aspect of mitochondrial biology is the exquisite regulation of calcium con-tent. Excessive levels of mitochondrial Ca+2 causes increased ROS production, mPTP open-ing and impairment of energetic function [1][29][30]. In addition, the disruption of mitochondrial contacts with membranes of the endoplasmic reticulum (ER) is a crucial event for mitochondrial integrity since function and structure are highly dependent on the flux of Ca+2 from and into the ER [31][32].
3. Mitochondrial Dynamics
Mitochondrial biogenesis is controlled by the transcriptional factor peroxisome proliferator activated receptor-gamma coactivator 1-alpha (PGC-1α) which is activated by di-rect interaction with NAD+-dependent deacetylase sirtuin 1 (SIRT1) [33] and phosphorylation by tAMP-activated protein kinase (AMPK) [34]. Then, phosphorylated PGC-1α is translocated into the nucleus where it promotes expression of the nuclear respiratory factors (NRFs) needed for further gene expression of mitochondrial proteins [35]. Among the mitochondrial proteins expressed are ETC protein subunits, fatty acid β-oxidation proteins and mitochondrial transcription factor A (mtTFA), which drive the transcription and replication of mtDNA. Additionally, NRFs also bind to promoter regions of genes coding for ROS scavengers [36][37].
The morphology, size and number of mitochondria are regulated by coordinated cycles of fusion and fission known as mitochondrial dynamics [38]. Mitochondrial fusion generates an interconnected mitochondrial network for the exchange of matrix contents and mtDNA molecules from healthy mitochondria donors to damaged mitochondria in order to reduce altered mtDNA. The main proteins with GTPase activity involved in mitochondrial fusion are optical atrophy 1 protein (OPA1), and mitofusins (Mfn1 and Mfn2) [39][40]. Mitochondrial fission generates smaller mitochondria without mtDNA replication by fragmentation and separation from the mitochondrial network followed by processing in the autophagosome [41]. Fission is modulated by GTPase dynamin related protein (DRP1), which is recruited from the cytosol to the OMM and interacts with fission 1 protein (Fis1) to stimulate mitochondrial fission [38][39] (Figure 1).
After mitochondrial fragmentation, the removal of altered mitochondria occurs by mitophagy, which is modulated by PTEN-induced kinase 1 (PINK1) and PARKIN RBR E3 ubiquitin-protein ligase (PARKIN) proteins [42][43]. PINK1 accumulates in the OMM in response to a reduction in MMP in dysfunctional mitochondria. Then, PARKIN is re-cruited from the cytosol to the OMM and promotes ubiquitination of mitochondrial proteins, leading to mitochondrial degradation [44][45]. Recently, it was observed that PINK1 accumulation and PARKIN recruitment are required to start mitophagy which is induced by slight oscillations in mitochondrial Ca+2 levels in human neuroblastoma SH-SY5Y cells [46]. In addition, PINK1 is also able to activate mitophagy directly without the participation of PARKIN by recruiting optineurin (OPTN) and nuclear dot protein 52 kDa (NDP52) [47]. In this case, PARKIN participates in further amplification of mitophagy in-duced by PINK1 [11] (Figure 1).
4. Mitochondrial Alterations Associated with NI&NDDs
Fragmented mitochondria with altered membrane structure have been found in the brain of patients with AD, which is a neurodegenerative disorder characterized by memory and learning impairment [48]. The dysregulation of mitochondrial Ca+2 content and accumulation of deformed mitochondria have been reported in animal models of HD [12][49][50], which is a dominant heritable pathology characterized by cognitive impairment, chorea, dystonia, and progressive loss of motor coordination [51]. mtDNA mutations affecting the ETC complexes have been reported in patients with MS [52], which is an autoimmune disease of the central nervous system (CNS) characterized by neuroinflammation, demyelination, and axonal damage [53]. In addition, several single nucleotide polymorphisms of mtDNA have been correlated with an increased risk of MS [54][55]. In comparison to healthy individuals, higher rates of mtDNA deletions were observed in the substantia nigra of autopsied brain samples from patients with PD [56], which is a neurodegenerative disease characterized by loss of dopaminergic neurons, leading to cognitive and motor alteration. Even though precise mechanisms determining how defective mitochondria promote the ND process are elusive, recent evidence has involved exacerbated mitochondrial ROS production in cellular toxicity and the promotion of aggregation and accumulation of toxic intracellular proteins. In turn, the accumulation of toxic proteins interferes with mitochondrial function, impairing energy production and maintaining an oxidative cellular unbalance that impacts the structure and function of the CNS [57][58][59][60]. In addition, the cytosolic release of mtDNA can induce the activation of the inflammatory response [61], leading to injury and functional impairment of the CNS. mtDNA from dysfunctional mitochondria also induces the activation of nucleotide-binding domain and leucine-rich repeat (NLR) pyrin domain containing 3 (NLRP3) inflammasome proteins, which is involved in cellular apoptosis [62]. Alternatively, the cytoplasmic release of proteins from dysfunctional mitochondria such as cytochrome c is able to promote cell loss in the CNS by apoptotic mechanisms [63][64]. Interestingly, a decreased anterograde and retrograde mitochondrial transport [65][66] has been involved in the subsequent structural alterations of axons and further morphological changes of mitochondria within the spinal cord of mice developing experimental autoimmune encephalomyelitis (EAE), an animal model of MS [65]. Thus, the impaired transport of mitochondria in neurons would limit the energetic supply needed to counteract demyelination and degenerative processes in the axonal terminal. Importantly, increasing mitochondrial transport from the neuronal cell body to the axon resulted in effective protection of demyelinated axons from degeneration [67].
4.1. Proteinopathies and Alteration of Mitochondrial Biology in NI&NDDs
Mitochondrial dysfunction associated with NI&NDDs is also facilitated by pathological accumulation of specific aberrant proteins as a result of nuclear gene mutations or abnormal protein processing leading to oligomeric and fibrillary aggregates. The anomalous accumulation of protein aggregates impacts mitochondrial structure and function either due to altered interaction with other subcellular organelles or dysregulation of processes involved in mitochondrial dynamics. The main protein aggregates related to proteinopathies are: amyloid β (Aβ) peptide and Tau protein in AD [68][69][70][71]; α-synuclein (α-syn) in PD [72][73][74], transactive response DNA-binding protein of 43 kDa (TDP-43) in AD and ALS [61][75][76]; Cu, Zn-superoxide dismutase (SOD1) in ALS [77][78]; and Huntingtin protein (Htt) in HD [50][79][80]. Table 1 summarizes the evidence relating these protein aggregates with mitochondrial dysfunction in ND diseases. α-Syn is a neuronal protein associated with the release of neurotransmitters and synaptic vesicles [81], and its misfolding and aggregation in structures referred to as Lewy bodies, particularly in dopaminergic neurons, is a hallmark of PD [81][82]. α-syn interaction with the mitochondrial structure is associated with an impairment of ETC activity, decreased MMP, mPTP opening and mitochondrial swelling as well as increased levels of mitochondrial ROS and neuron cell death [73][74]. In addition, α-syn interferes with the mitochondrial contacts with ER, leading to the disruption of Ca+2 flux and a reduction of ATP production [83]. Additionally, α-syn association with mitochondria results in downregulation of the predominant SIRT in the mitochondria (SIRT3) [72], which is a molecule that protects mitochondrial integrity and energetic function [84]. SIRT3 reduction is also accompanied by an increased expression of the fission protein DRP1 in neural cells and brain tissue of mice expressing α-syn [73]. α-Syn is also upregulated in the neurons and glia of demyelinating lesions in the spinal cord of mice developing EAE [85][86]. Moreover, the levels of α-syn in the cerebrospinal fluid of MS patients correlates with disease disability, suggesting a participation of α-syn in demyelinating and NI pathologies [87]. The intracellular accumulation of aggregates of Aβ peptide and hyperphosphorylated protein Tau are biochemical characteristics of AD [71][88][89]. The Aβ peptide derives from amyloid precursor protein (APP) in the mitochondria–ER contacts through sequential processing by BACE1 (β-site APP cleavage enzyme 1) and γ-secretase [90]. Aβ interacts with mitochondrial molecules and structures, which results in ROS production, mitochondrial dysfunction, and subsequent cell damage [91][92][93][94]. In addition, Aβ alters mitochondrial morphology and fragmentation by increasing DRP1 and reducing Mfn1 levels in cellular models [68][94]. Moreover, the mitochondrial fission promoted by Aβ occurs through O-GlcNAcylation of DRP1 in both neuronal cell lines and primary cultured neurons [69]. Interestingly, the activated form of DRP1 by O-GlcNAcylation is also found in the brains of mice in an AD mouse model [69]. Tau is a microtubule-binding protein in neurons, and its abnormal processing and aggregation by hyperphosphorylation promotes dissociation of preformed microtubules, interaction with mitochondrial ETC complexes, reduction of ATP production, and neuronal death [71][93]. In addition, hyperphosphorylated Tau interacts with VDAC1 mitochondrial protein promoting the alteration of energetic functions, Ca+2 homeostasis, and oxidative balance [71][91]. In addition, Tau alters mitochondrial fission and mitophagy by interacting with DRP1 and PARKIN proteins, respectively, in both patients and transgenic mouse models of AD [70][93]. TDP-43 is an essential ribonucleoprotein that can also form toxic cytosolic aggregates in AD. Diverse mutated forms of TPD-43 have been localized as aggregates in the mitochondria of mouse models and patients with familial ALS and are associated with structural and functional alterations in mitochondria [61][95][96]. Consistently, the inhibition of mitochondrial localization of TDP-43 restored mitochondrial function and ameliorated motor and cognitive deficits in ALS and AD models [75][76][97]. In HD, the generation of a mutated huntingtin protein (mHTT) by abnormal expansion of a CAG polyglutamine trinucleotide [98] impacted mitochondrial function and subsequent ND processes [50][79][80]. The enzyme SOD1 is mainly a cytosolic molecule, but mutated forms of SOD1 have been localized in aggregates associated with mitochondria in transgenic mouse models and patients with familial ALS [77][78]. Interestingly, the pharmacological reduction of misfolded SOD1 restored the structural integrity of mitochondria, reduced degeneration of motor neurons, and attenuated motor deficits in a transgenic ALS mouse model [99].
Table 1. Protein aggregates related to mitochondrial dysfunction in models of neurodegenerative diseases.
| Protein Aggregates |
Disease Model | Effect on Mitochondria | Refs |
|---|---|---|---|
| α-synuclein | PD | ↑mitochondrial ROS levels, ↓ ETC activity, ↓stability of mitochondrial membranes, ↑mPTP opening, ↓mitochondria-ER contacts, ↑DRP1 and ↓mitochondrial SIRT3 levels (a protective molecule of mitochondrial integrity and energetic function [84]) | [72][73][74][83][100] |
| Amyloid b | AD | ↑mitochondrial ROS levels, ↑mitochondrial fission (↓Mfn1, ↑DRP1 levels and ↑O-GlcNAcylation of DRP1) | [68][69][91] [92][94] |
| Tau | AD | ↑microtubule dissociation, ↑mitochondrial ROS levels, ↓ATP production, ↑mitochondrial fission and ↓mitophagy (interaction with DRP1 and PARKIN proteins) |
[70][71][93] |
| Transactive response DNA-binding protein of 43 kDa (TDP-43) |
AD, ALS | ↑mitochondrial ROS levels, ↓stability of mitochondrial structure, ↑mPTP opening |
[61][75][95][96][97] |
| Huntingtin | HD | ↑mitochondrial ROS levels, ↓stability of mitochondrial structure, ↑mPTP opening, ↑mitochondrial fission by activation of DRP1, ↓mitophagy, ↑disruption Ca+2 flux between ER and mitochondria | [49][79] [80][101] |
| Superoxide dismutase |
ALS | ↓mitophagy (by arresting optineurin protein), ↓stability of mitochondria structure, ↓flux of protein from and to the mitochondria | [102][103][104][105] |
ROS: reactive oxygen species, SIRT3: sirtuin 3, DRP1: dynamin related protein, α-syn: α-synuclein, ETC: electron transport chain, ER: endoplasmic reticulum, mPTP: mitochondrial permeability transition pore, Mfn1: mitofusin 1, PARKIN: PARKIN RBR E3 ubiquitin-protein ligase, PD: Parkinson’s disease, AD: Alzheimer’s disease, ALS: amyotrophic lateral sclerosis, HD: Huntington’s disease.
4.2. Alteration of Mitochondrial Dynamics in NI&NDDs
Mitochondrial dysfunction associated with NI&NDDs is also characterized by the altered activity of key proteins involved in mitochondrial dynamics. Diverse studies performed in cellular and animal models of NI&NDDs as well as in postmortem brain tissue of patients with NI&NDDs have shown increased activity of fission proteins such as DRP1 and FIS1 and in some cases reduced levels of Mfn and OPA proteins [50][69][80][94][101][106]. Interestingly, reversing DRP1 activation by pharmacological or genetic inhibition reduced mitochondrial fission and cell death in cellular and animal models of ND diseases [107][108][109]. Alternatively, increasing the mitochondrial fusion through overexpression of Mfn2 restored mitochondrial dynamics and attenuated neural damage and motor deficits in NI&NDDs [110].
In addition, impaired mitophagy has been observed in several studies with animal models of NI&NDDs [46][79][111]. Dysfunctional mitophagy has been associated with altered aspects of PINK and PARKIN function; such as their inactivation, deficient expression, suppression of mitochondria calcium signaling hampering recruitment of PINK and PARKIN to the mitochondria [46], or gene mutations affecting these proteins. Mutations in PINK and PARKIN genes have been related to the origin of familial PD, which can represent around 10% of the diagnosed forms of PD [112][113][114]. Interestingly, normalizing PINK and PARKIN activity through chemical agents restored mitophagy and cognitive impairment in ND models [13][115]. Additionally, PINK1 overexpression rescued mitochondrial dysfunction and restored the removal of defective mitochondria in transgenic animal models of AD and HD [11][12][116].
Recently, a rat model of PD induced by direct intracerebroventricular injection of 6-hydroxydopamine (6-OHDA) exhibited accumulated defective mitochondria as spheroids in dopaminergic neurons by unfinished mitophagy. Interestingly, altered mitochondria were transferred to surrounding astrocytes in order to complete the remaining steps of mitophagy [117]. The cellular expulsion of damaged mitochondria has also been observed in a PD model of neuronal cells treated with rotenone and in fibroblasts and cerebrospinal fluid samples from PD patients carrying a PARKIN mutation [118]. A self-destructive process of mitochondrial removal referred to as mitoautophagy has been proposed to operate in the motor neurons of an ALS transgenic mouse model. Unlike classical mitophagy, mitoautophagy produces mitochondrial degradation without the participation of lysosomes or autophagasomes [105].
Decreased mitochondrial biogenesis is observed in both patients and animal models of ND diseases [119][120]. The impaired process is characterized by the altered expression of PGC-1α, which is the main molecule involved in mitochondrial biogenesis. PGC-1α expression is significantly reduced in the brain tissue of HD mice [80] as well as in postmortem brain tissue of AD [121] and MS patients [122]. Additionally, PGC-1α has been found post-translationally inactivated by phosphorylation in the neurons of spinal cords from EAE mice [14]. Consistently, mice overexpressing neuronal PGC-1α showed an increased number of active mitochondria with enhanced respiratory capacity and a significantly better recovery of clinical disability and neurodegeneration induced by EAE compared to wild-type controls [14].
4.3. Energy Impairment Associated with Mitochondrial Dysfunction in NI&NDDs
A common hallmark of different NI&NDDs is the bioenergetic deficit due to mitochondrial dysfunction. ETC activity and ATP production declines in mitochondria in early stages of AD and ALS [123][124] and an altered brain energy metabolism, evidenced by a reduced neuronal glucose uptake, is associated with the progression of neurodegeneration and frequently manifests before symptomatic onset in AD, PD, ALS, and HD [125][126][127][128][129]. In addition, brain glucose hypometabolism correlates with subsequent patterns of motor and cognitive deficits along with pathology progression [125][130][131]. Reduced glucose uptake in the brain of patients with ND diseases associates with brain insulin receptor desensitization, as evidenced by the significant reduction and deterioration of insulin receptors in AD [132][133]. Interestingly, insulin regulates the function of mitochondria by upregulating the ETC complex proteins [134] and brain insulin resistance has been related to mitochondrial dysfunction and promotion of PD [135]. Consistently, evidence (see Section 6) shows that compounds that promote uptake of glucose as well as insulin sensitizers restore mitochondrial function and ameliorate cognitive and motor disturbances in ND mouse models. Therefore, an energy impairment promoted by the hypometabolism of glucose and dysfunctional mitochondria contributes to ND diseases [136][137][138].
4.4. Oxidative Stress Associated with Mitochondrial Dysfunction in NI&NDDs
Impaired operation of ETC is observed in the mitochondria of neurodegenerative tissues leading to increased ROS production and oxidative stress. The brain is very sensitive to redox impairment due to the high content of polyunsaturated fatty acids. Therefore, the brain is prone to suffer further oxidation events due to the presence of transition metals, such as iron and copper, putting mitochondria in a circular loop of damage and leading to more defective mitochondria [9][10]. Thus, dysfunctional mitochondria can exacerbate the oxidative environment in ND diseases. In turn, oxidative conditions severely affect the machinery involved in energy production exacerbating ROS production in mitochondria. For example, the expression of mtDNA genes required for OXPHOS and ETC function are significantly reduced in postmortem brain tissue of AD and PD [121] as well as in MS patients and in motor neurons of the spinal cords of EAE mice [52][57][139][140]. In these cases, reduced mitochondrial function is accompanied by an augmented oxidative response which often precedes more severe signs of biochemical and clinical defects in memory and neurodegeneration [57][58]. Studies have determined reduced activity of the complex II enzyme succinate dehydrogenase (SDH) and a decreased complex II-III activity in the basal ganglia of HD patients along with a decrease in complex IV activity in HD striatum. 3-nitropropionic acid (NPA), an inhibitor of SDH, causes mitochondrial damage leading to an increase in electron leakage from the mitochondria, production of ROS and nitrogen species, and depletion of antioxidant defenses. Interestingly, 3-NPA in rodents causes striatal degeneration and impairment in locomotor activity, body weight, and cognitive deficits that closely mimic symptoms seen in HD [141][142]. PD induced in mice or primates by diverse compounds such as 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), rotenone or 6-OHDA produces loss of dopaminergic neurons by inhibition of ETC in the mitochondria and exacerbated generation of ROS [143][144][145][146]. Impaired mitochondrial production of ATP in motor neurons in mouse models and patients with ALS is accompanied by massive oxidative damage prior to the manifestation of clinical symptoms or at early stages of disease [60][147][148].The oxidative stress can also induce mitochondrial dysfunction by affecting mitochondrial dynamics. Reactive nitrogen species can activate DRP1, leading to mitochondrial fragmentation and ND damage in cellular and animal models of AD and MS [148][149]. Interestingly, treatment with chemical antioxidants reverted the defective operation of mitochondrial OXPHOS in cultured fibroblasts from ALS patients [16]. Thus, counteracting the oxidative stress associated with mitochondria dysfunction could be an important therapeutic strategy for tackling ND processes.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13122055
References
- Bauer, T.M.; Murphy, E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020, 126, 280–293.
- Friedman, J.R.; Nunnari, J. Mitochondrial Form and Function. Nature 2014, 505, 335–343.
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495.
- Archer, S.L. Mitochondrial Dynamics—Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med. 2013, 369, 2236–2251.
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 235–259.
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 2018, 62, 341–360.
- Yan, X.; Wang, B.; Hu, Y.; Wang, S.; Zhang, X. Abnormal Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 138.
- Panchal, K.; Tiwari, A.K. Mitochondrial Dynamics, a Key Executioner in Neurodegenerative Diseases. Mitochondrion 2019, 47, 151–173.
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785.
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible To Oxidative Stress. Redox Biol. 2018, 15, 490–503.
- Du, F.; Yu, Q.; Yan, S.; Hu, G.; Lue, L.F.; Walker, D.G.; Wu, L.; Yan, S.F.; Tieu, K.; Yan, S.S. PINK1 Signalling Rescues Amyloid Pathology and Mitochondrial Dysfunction in Alzheimer’s Disease. Brain 2017, 140, 3233–3251.
- Khalil, B.; El Fissi, N.; Aouane, A.; Cabirol-Pol, M.J.; Rival, T.; Liévens, J.C. PINK1-Induced Mitophagy Promotes Neuroprotection in Huntington’s Disease. Cell Death Dis. 2015, 6, e1617.
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy Inhibits Amyloid-β and Tau Pathology and Reverses Cognitive Deficits in Models of Alzheimer’s Disease. Nat. Neurosci. 2019, 22, 401–412.
- Rosenkranz, S.C.; Shaposhnykov, A.A.; Träger, S.; Engler, J.B.; Witte, M.E.; Roth, V.; Vieira, V.; Paauw, N.; Bauer, S.; Schwencke-Westphal, C.; et al. Enhancing Mitochondrial Activity in Neurons Protects against Neurodegeneration in a Mouse Model of Multiple Sclerosis. eLife 2021, 10, e61798.
- Aman, Y.; Ryan, B.; Torsetnes, S.B.; Knapskog, A.-B.; Watne, L.O.; McEwan, W.A.; Fang, E.F. Enhancing mitophagy as a therapeutic approach for neurodegenerative diseases. In Metabolic and Bioenergetic Drivers of Neurodegenerative Disease: Treating Neurodegenerative Diseases as Metabolic Diseases, 1st ed.; Söderbom, G., Esterline, R., Oscarsson, J., Mattson, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 155, pp. 169–202.
- Debska-Vielhaber, G.; Miller, I.; Peeva, V.; Zuschratter, W.; Walczak, J.; Schreiber, S.; Petri, S.; Machts, J.; Vogt, S.; Szczepanowska, J.; et al. Impairment of Mitochondrial Oxidative Phosphorylation in Skin Fibroblasts of SALS and FALS Patients Is Rescued by in Vitro Treatment with ROS Scavengers. Exp. Neurol. 2021, 339, 113620.
- Nesci, S. The Mitochondrial Permeability Transition Pore in Cell Death: A Promising Drug Binding Bioarchitecture. Med. Res. Rev. 2020, 40, 811–817.
- Cesura, A.M.; Pinard, E.; Schubenel, R.; Goetschy, V.; Friedlein, A.; Langen, H.; Polcic, P.; Forte, M.A.; Bernardi, P.; Kemp, J.A. The Voltage-Dependent Anion Channel Is the Target for a New Class of Inhibitors of the Mitochondrial Permeability Transition Pore. J. Biol. Chem. 2003, 278, 49812–49818.
- Gammage, P.A.; Frezza, C. Mitochondrial DNA: The Overlooked Oncogenome? BMC Biol. 2019, 17, 53.
- Fullerton, M.; McFarland, R.; Taylor, R.W.; Alston, C.L. The Genetic Basis of Isolated Mitochondrial Complex II Deficiency. Mol. Genet. Metab. 2020, 131, 53–65.
- Madeira, V.M.C. Overview of Mitochondrial Bioenergetics. Methods Mol. Biol. 2018, 1782, 1–6.
- Nath, S.; Villadsen, J. Oxidative Phosphorylation Revisited. Biotechnol. Bioeng. 2015, 112, 429–437.
- Brand, M.D. Mitochondrial Generation of Superoxide and Hydrogen Peroxide as the Source of Mitochondrial Redox Signaling. Free Radic. Biol. Med. 2016, 100, 14–31.
- Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial Production of Reactive Oxygen Species. Biochemistry 2013, 78, 1490–1511.
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13.
- Bleier, L.; Dröse, S. Superoxide Generation by Complex III: From Mechanistic Rationales to Functional Consequences. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 1320–1331.
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 57.
- Woo, J.; Cho, H.; Seol, Y.; Kim, S.H.; Park, C.; Yousefian-Jazi, A.; Hyeon, S.J.; Lee, J.; Ryu, H. Power Failure of Mitochondria and Oxidative Stress in Neurodegeneration and Its Computational Models. Antioxidants 2021, 10, 229.
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478.
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833.
- Lee, K.S.; Huh, S.; Lee, S.; Wu, Z.; Kim, A.K.; Kang, H.Y.; Lu, B. Altered ER-Mitochondria Contact Impacts Mitochondria Calcium Homeostasis and Contributes to Neurodegeneration in Vivo in Disease Models. Proc. Natl. Acad. Sci. USA 2018, 115, E8844–E8853.
- Fan, Y.; Simmen, T. Mechanistic Connections between Endoplasmic Reticulum (ER) Redox Control and Mitochondrial Metabolism. Cells 2019, 8, 1071.
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 Functionally Interacts with the Metabolic Regulator and Transcriptional Coactivator PGC-1α. J. Biol. Chem. 2005, 280, 16456–16460.
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD + Metabolism and SIRT1 Activity. Nature 2009, 458, 1056–1060.
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial Biogenesis in Neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029.
- Gureev, A.P.; Popov, V.N. Nrf2/ARE Pathway as a Therapeutic Target for the Treatment of Parkinson Diseases. Neurochem. Res. 2019, 44, 2273–2279.
- Goodfellow, M.J.; Borcar, A.; Proctor, J.L.; Greco, T.; Rosenthal, R.E.; Fiskum, G. Transcriptional Activation of Antioxidant Gene Expression by Nrf2 Protects against Mitochondrial Dysfunction and Neuronal Death Associated with Acute and Chronic Neurodegeneration. Exp. Neurol. 2020, 328, 113247.
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial Fission and Fusion: A Dynamic Role in Aging and Potential Target for Age-Related Disease. Mech. Ageing Dev. 2020, 186, 111212.
- Bertholet, A.M.; Delerue, T.; Millet, A.M.; Moulis, M.F.; David, C.; Daloyau, M.; Arnauné-Pelloquin, L.; Davezac, N.; Mils, V.; Miquel, M.C.; et al. Mitochondrial Fusion/Fission Dynamics in Neurodegeneration and Neuronal Plasticity. Neurobiol. Dis. 2016, 90, 3–19.
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021, 31, 62–74.
- Mao, K.; Klionsky, D.J. Mitochondrial Fission Facilitates Mitophagy in Saccharomyces Cerevisiae. Autophagy 2013, 9, 1900–1901.
- Lou, G.; Palikaras, K.; Lautrup, S.; Scheibye-Knudsen, M.; Tavernarakis, N.; Fang, E.F. Mitophagy and Neuroprotection. Trends Mol. Med. 2020, 26, 8–20.
- Montava-Garriga, L.; Ganley, I.G. Outstanding Questions in Mitophagy: What We Do and Do Not Know. J. Mol. Biol. 2020, 432, 206–230.
- Tanaka, K. The PINK1–Parkin Axis: An Overview. Neurosci. Res. 2020, 159, 9–15.
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN Signalling in Neurodegeneration and Neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189.
- Yu, Z.; Wang, H.; Tang, W.; Wang, S.; Tian, X.; Zhu, Y.; He, H. Mitochondrial Ca2+ Oscillation Induces Mitophagy Initiation through the PINK1-Parkin Pathway. Cell Death Dis. 2021, 12, 632.
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The Ubiquitin Kinase PINK1 Recruits Autophagy Receptors to Induce Mitophagy. Nature 2015, 524, 309–314.
- Xie, H.; Guan, J.S.; Borrelli, L.A.; Xu, J.; Serrano-Pozo, A.; Bacskai, B.J. Mitochondrial Alterations near Amyloid Plaques in an Alzheimer’s Disease Mouse Model. J. Neurosci. 2013, 33, 17042–17051.
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial dysfunction in huntington’s disease. In Polyglutamine Disorders, 1st ed.; Nóbrega, C., Pereira de Almeida, L., Eds.; Springer: Cham, Switzerland; New York, NY, USA, 2018; Volume 1049, pp. 59–83.
- Cherubini, M.; Lopez-Molina, L.; Gines, S. Mitochondrial Fission in Huntington’s Disease Mouse Striatum Disrupts ER-Mitochondria Contacts Leading to Disturbances in Ca2+ Efflux and Reactive Oxygen Species (ROS) Homeostasis. Neurobiol. Dis. 2020, 136, 104741.
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34.
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA Deletions and Neurodegeneration in Multiple Sclerosis. Ann. Neurol. 2011, 69, 481–492.
- Simkins, T.J.; Duncan, G.J.; Bourdette, D. Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications. Curr. Neurol. Neurosci. Rep. 2021, 21, 654284.
- Yu, X.; Koczan, D.; Sulonen, A.M.; Akkad, D.A.; Kroner, A.; Comabella, M.; Costa, G.; Corongiu, D.; Goertsches, R.; Camina-Tato, M.; et al. MtDNA Nt13708A Variant Increases the Risk of Multiple Sclerosis. PLoS ONE 2008, 3, e1530.
- Andalib, S.; Emamhadi, M.; Yousefzadeh-Chabok, S.; Salari, A.; Sigaroudi, A.E.; Vafaee, M.S. MtDNA T4216C Variation in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Acta Neurol. Belg. 2016, 116, 439–443.
- Dölle, C.; Flønes, I.; Nido, G.S.; Miletic, H.; Osuagwu, N.; Kristoffersen, S.; Lilleng, P.K.; Larsen, J.P.; Tysnes, O.B.; Haugarvoll, K.; et al. Defective Mitochondrial DNA Homeostasis in the Substantia Nigra in Parkinson Disease. Nat. Commun. 2016, 7, 13548.
- Gonzalo, H.; Nogueras, L.; Gil-Sánchez, A.; Hervás, J.V.; Valcheva, P.; González-Mingot, C.; Martin-Gari, M.; Canudes, M.; Peralta, S.; Solana, M.J.; et al. Impairment of Mitochondrial Redox Status in Peripheral Lymphocytes of Multiple Sclerosis Patients. Front. Neurosci. 2019, 13, 938.
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial Bioenergetic Deficit Precedes Alzheimer’s Pathology in Female Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 14670–14675.
- Damiano, M.; Diguet, E.; Malgorn, C.; D’Aurelio, M.; Galvan, L.; Petit, F.; Benhaim, L.; Guillermier, M.; Houitte, D.; Dufour, N.; et al. A Role of Mitochondrial Complex II Defects in Genetic Models of Huntington’s Disease Expressing N-Terminal Fragments of Mutant Huntingtin. Hum. Mol. Genet. 2013, 22, 3869–3882.
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 6352.
- Yu, C.H.; Davidson, S.; Harapas, C.R.; Hilton, J.B.; Mlodzianoski, M.J.; Laohamonthonkul, P.; Louis, C.; Low, R.R.J.; Moecking, J.; De Nardo, D.; et al. TDP-43 Triggers Mitochondrial DNA Release via MPTP to Activate CGAS/STING in ALS. Cell 2020, 183, 636-649.e18.
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414.
- Pinto, M.; Vempati, U.D.; Diaz, F.; Peralta, S.; Moraes, C.T. Ablation of Cytochrome c in Adult Forebrain Neurons Impairs Oxidative Phosphorylation Without Detectable Apoptosis. Mol. Neurobiol. 2019, 56, 3722–3735.
- Clayton, R.; Clark, J.B.; Sharpe, M. Cytochrome c Release from Rat Brain Mitochondria Is Proportional to the Mitochondrial Functional Deficit: Implications for Apoptosis and Neurodegenerative Disease. J. Neurochem. 2005, 92, 840–849.
- Sadeghian, M.; Mastrolia, V.; Rezaei Haddad, A.; Mosley, A.; Mullali, G.; Schiza, D.; Sajic, M.; Hargreaves, I.; Heales, S.; Duchen, M.R.; et al. Mitochondrial Dysfunction Is an Important Cause of Neurological Deficits in an Inflammatory Model of Multiple Sclerosis. Sci. Rep. 2016, 6, 33249.
- Sorbara, C.D.; Wagner, N.E.; Ladwig, A.; Nikić, I.; Merkler, D.; Kleele, T.; Marinković, P.; Naumann, R.; Godinho, L.; Bareyre, F.M.; et al. Pervasive Axonal Transport Deficits in Multiple Sclerosis Models. Neuron 2014, 84, 1183–1190.
- Licht-Mayer, S.; Campbell, G.R.; Canizares, M.; Mehta, A.R.; Gane, A.B.; McGill, K.; Ghosh, A.; Fullerton, A.; Menezes, N.; Dean, J.; et al. Enhanced Axonal Response of Mitochondria to Demyelination Offers Neuroprotection: Implications for Multiple Sclerosis. Acta Neuropathol. 2020, 140, 143–167.
- Panes, J.D.; Godoy, P.A.; Silva-Grecchi, T.; Celis, M.T.; Ramirez-Molina, O.; Gavilan, J.; Muñoz-Montecino, C.; Castro, P.A.; Moraga-Cid, G.; Yévenes, G.E.; et al. Changes in PGC-1α/SIRT1 Signaling Impact on Mitochondrial Homeostasis in Amyloid-Beta Peptide Toxicity Model. Front. Pharmacol. 2020, 11, 709.
- Park, S.J.; Bae, J.E.; Jo, D.S.; Kim, J.B.; Park, N.Y.; Fang, J.; Jung, Y.K.; Jo, D.G.; Cho, D.H. Increased O-GlcNAcylation of Drp1 by Amyloid-Beta Promotes Mitochondrial Fission and Dysfunction in Neuronal Cells. Mol. Brain 2021, 14, 6.
- Cummins, N.; Tweedie, A.; Zuryn, S.; Bertran-Gonzalez, J.; Götz, J. Disease-associated Tau Impairs Mitophagy by Inhibiting Parkin Translocation to Mitochondria. EMBO J. 2019, 38, e99360.
- Szabo, L.; Eckert, A.; Grimm, A. Insights into Disease-Associated Tau Impact on Mitochondria. Int. J. Mol. Sci. 2020, 21, 6344.
- Park, J.H.; Burgess, J.D.; Faroqi, A.H.; Demeo, N.N.; Fiesel, F.C.; Springer, W.; Delenclos, M.; McLean, P.J. Alpha-Synuclein-Induced Mitochondrial Dysfunction Is Mediated via a Sirtuin 3-Dependent Pathway. Mol. Neurodegener. 2020, 15, 5.
- Reeve, A.K.; Ludtmann, M.H.R.; Angelova, P.R.; Simcox, E.M.; Horrocks, M.H.; Klenerman, D.; Gandhi, S.; Turnbull, D.M.; Abramov, A.Y. Aggregated α-Synuclein and Complex I Deficiency: Exploration of Their Relationship in Differentiated Neurons. Cell Death Dis. 2015, 6, e1820.
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A.V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-Synuclein Oligomers Interact with ATP Synthase and Open the Permeability Transition Pore in Parkinson’s Disease. Nat. Commun. 2018, 9, 2293.
- Wang, W.; Wang, L.; Lu, J.; Siedlak, S.L.; Fujioka, H.; Liang, J.; Jiang, S.; Ma, X.; Jiang, Z.; Da Rocha, E.L.; et al. The Inhibition of TDP-43 Mitochondrial Localization Blocks Its Neuronal Toxicity. Nat. Med. 2016, 22, 869–878.
- Gao, J.; Wang, L.; Gao, C.; Arakawa, H.; Perry, G.; Wang, X. TDP-43 Inhibitory Peptide Alleviates Neurodegeneration and Memory Loss in an APP Transgenic Mouse Model for Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165580.
- Vijayvergiya, C.; Beal, M.F.; Buck, J.; Manfredi, G. Mutant Superoxide Dismutase 1 Forms Aggregates in the Brain Mitochondrial Matrix of Amyotrophic Lateral Sclerosis Mice. J. Neurosci. 2005, 25, 2463–2470.
- Higgins, C.M.J.; Jung, C.; Ding, H.; Xu, Z. Mutant Cu, Zn Superoxide Dismutase That Causes Motoneuron Degeneration Is Present in Mitochondria in the CNS. J. Neurosci. 2002, 22, RC215.
- Franco-Iborra, S.; Plaza-Zabala, A.; Montpeyo, M.; Sebastian, D.; Vila, M.; Martinez-Vicente, M. Mutant HTT (Huntingtin) Impairs Mitophagy in a Cellular Model of Huntington Disease. Autophagy 2021, 17, 672–689.
- Shirendeb, U.P.; Calkins, M.J.; Manczak, M.; Anekonda, V.; Dufour, B.; McBride, J.L.; Mao, P.; Reddy, P.H. Mutant Huntingtin’s Interaction with Mitochondrial Protein Drp1 Impairs Mitochondrial Biogenesis and Causes Defective Axonal Transport and Synaptic Degeneration in Huntington’s Disease. Hum. Mol. Genet. 2012, 21, 406–420.
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091.
- Manzanza, N.; de Oliveira Manzanza, N.; Sedlackova, L.; Kalaria, R.N. Alpha-Synuclein Post-Translational Modifications: Implications for Pathogenesis of Lewy Body Disorders. Front. Aging Neurosci. 2021, 13, 690293.
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C.J. α-Synuclein Binds to the ER–Mitochondria Tethering Protein VAPB to Disrupt Ca2+ Homeostasis and Mitochondrial ATP Production. Acta Neuropathol. 2017, 134, 129–149.
- Lombard, D.B.; Zwaans, B.M.M. SIRT3: As Simple as It Seems? Gerontology 2014, 60, 56–64.
- Lieberknecht, V.; Junqueira, S.C.; Cunha, M.P.; Barbosa, T.A.; de Souza, L.F.; Coelho, I.S.; Santos, A.R.S.; Rodrigues, A.L.S.; Dafré, A.L.; Dutra, R.C. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Mol. Neurobiol. 2017, 54, 1033–1045.
- Papadopoulos, D.; Ewans, L.; Pham-Dinh, D.; Knott, J.; Reynolds, R. Upregulation of α-Synuclein in Neurons and Glia in Inflammatory Demyelinating Disease. Mol. Cell. Neurosci. 2006, 31, 597–612.
- Wang, H.; Wang, K.; Xu, W.; Wang, C.; Qiu, W.; Zhong, X.; Dai, Y.; Wu, A.; Hu, X. Cerebrospinal Fluid α-Synuclein Levels Are Elevated in Multiple Sclerosis and Neuromyelitis Optica Patients during Replase. J. Neurochem. 2012, 122, 19–23.
- Sun, B.L.; Li, W.W.; Zhu, C.; Jin, W.S.; Zeng, F.; Liu, Y.H.; Bu, X.L.; Zhu, J.; Yao, X.Q.; Wang, Y.J. Clinical Research on Alzheimer’s Disease: Progress and Perspectives. Neurosci. Bull. 2018, 34, 1111–1118.
- Sivanesan, S.; Chang, E.; Howell, M.D.; Rajadas, J. Amyloid Protein Aggregates: New Clients for Mitochondrial Energy Production in the Brain? FEBS J. 2020, 287, 3386–3395.
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756.
- Manczak, M.; Reddy, P.H. Abnormal Interaction of VDAC1 with Amyloid Beta and Phosphorylated Tau Causes Mitochondrial Dysfunction in Alzheimer’s Disease. Hum. Mol. Genet. 2012, 21, 5131–5146.
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD Directly Links Aβ to Mitochondrial Toxicity in Alzheimer’s Disease. Science 2004, 304, 448–452.
- Manczak, M.; Reddy, P.H. Abnormal Interaction between the Mitochondrial Fission Protein Drp1 and Hyperphosphorylated Tau in Alzheimer’s Disease Neurons: Implications for Mitochondrial Dysfunction and Neuronal Damage. Hum. Mol. Genet. 2012, 21, 2538–2547.
- Manczak, M.; Calkins, M.J.; Reddy, P.H. Impaired Mitochondrial Dynamics and Abnormal Interaction of Amyloid Beta with Mitochondrial Protein Drp1 in Neurons from Patients with Alzheimer’s Disease: Implications for Neuronal Damage. Hum. Mol. Genet. 2011, 20, 2495–2509.
- Gautam, M.; Jara, J.H.; Kocak, N.; Rylaarsdam, L.E.; Kim, K.D.; Bigio, E.H.; Hande Özdinler, P. Mitochondria, ER, and Nuclear Membrane Defects Reveal Early Mechanisms for Upper Motor Neuron Vulnerability with Respect to TDP-43 Pathology. Acta Neuropathol. 2019, 137, 47–69.
- Magrané, J.; Cortez, C.; Gan, W.B.; Manfredi, G. Abnormal Mitochondrial Transport and Morphology Are Common Pathological Denominators in SOD1 and TDP43 ALS Mouse Models. Hum. Mol. Genet. 2014, 23, 1413–1424.
- Wang, W.; Arakawa, H.; Wang, L.; Okolo, O.; Siedlak, S.L.; Jiang, Y.; Gao, J.; Xie, F.; Petersen, R.B.; Wang, X. Motor-Coordinative and Cognitive Dysfunction Caused by Mutant TDP-43 Could Be Reversed by Inhibiting Its Mitochondrial Localization. Mol. Ther. 2017, 25, 127–139.
- Yang, H.; Yang, S.; Jing, L.; Huang, L.; Chen, L.; Zhao, X.; Yang, W.; Pan, Y.; Yin, P.; Qin, Z.S.; et al. Truncation of Mutant Huntingtin in Knock-in Mice Demonstrates Exon1 Huntingtin Is a Key Pathogenic Form. Nat. Commun. 2020, 11, 2582.
- Genç, B.; Gautam, M.; Gözütok, Ö.; Dervishi, I.; Sanchez, S.; Goshu, G.M.; Koçak, N.; Xie, E.; Silverman, R.B.; Özdinler, P.H. Improving Mitochondria and ER Stability Helps Eliminate Upper Motor Neuron Degeneration That Occurs Due to MSOD1 Toxicity and TDP-43 Pathology. Clin. Transl. Med. 2021, 11, e336.
- Gilmozzi, V.; Gentile, G.; Castelo Rueda, M.P.; Hicks, A.A.; Pramstaller, P.P.; Zanon, A.; Lévesque, M.; Pichler, I. Interaction of Alpha-Synuclein With Lipids: Mitochondrial Cardiolipin as a Critical Player in the Pathogenesis of Parkinson’s Disease. Front. Neurosci. 2020, 14, 578993.
- Song, W.; Chen, J.; Petrilli, A.; Liot, G.; Klinglmayr, E.; Zhou, Y.; Poquiz, P.; Tjong, J.; Pouladi, M.A.; Hayden, M.R.; et al. Mutant Huntingtin Binds the Mitochondrial Fission GTPase Dynamin-Related Protein-1 and Increases Its Enzymatic Activity. Nat. Med. 2011, 17, 377–383.
- Li, Q.; Velde, C.V.; Israelson, A.; Xie, J.; Bailey, A.O.; Dong, M.Q.; Chun, S.J.; Roy, T.; Winer, L.; Yates, J.R.; et al. ALS-Linked Mutant Superoxide Dismutase 1 (SOD1) Alters Mitochondrial Protein Composition and Decreases Protein Import. Proc. Natl. Acad. Sci. USA 2010, 107, 21146–21151.
- Israelson, A.; Arbel, N.; Da Cruz, S.; Ilieva, H.; Yamanaka, K.; Shoshan-Barmatz, V.; Cleveland, D.W. Misfolded Mutant SOD1 Directly Inhibits VDAC1 Conductance in a Mouse Model of Inherited ALS. Neuron 2010, 67, 575–587.
- Tak, Y.J.; Park, J.H.; Rhim, H.; Kang, S. ALS-Related Mutant SOD1 Aggregates Interfere with Mitophagy by Sequestering the Autophagy Receptor Optineurin. Int. J. Mol. Sci. 2020, 21, 7525.
- Gautam, M.; Xie, E.F.; Kocak, N.; Ozdinler, P.H. Mitoautophagy: A Unique Self-Destructive Path Mitochondria of Upper Motor Neurons With TDP-43 Pathology Take, Very Early in ALS. Front. Cell. Neurosci. 2019, 13, 489.
- Filichia, E.; Hoffer, B.; Qi, X.; Luo, Y. Inhibition of Drp1 Mitochondrial Translocation Provides Neural Protection in Dopaminergic System in a Parkinson’s Disease Model Induced by MPTP. Sci. Rep. 2016, 6, 32656.
- Baek, S.H.; Park, S.J.; Jeong, J.I.; Kim, S.H.; Han, J.; Kyung, J.W.; Baik, S.H.; Choi, Y.; Choi, B.Y.; Park, J.S.; et al. Inhibition of Drp1 Ameliorates Synaptic Depression, Aβ Deposition, and Cognitive Impairment in an Alzheimer’s Disease Model. J. Neurosci. 2017, 37, 5099–5110.
- Guo, X.; Disatnik, M.H.; Monbureau, M.; Shamloo, M.; Mochly-Rosen, D.; Qi, X. Inhibition of Mitochondrial Fragmentation Diminishes Huntington’s Disease-Associated Neurodegeneration. J. Clin. Investig. 2013, 123, 5371–5388.
- Rappold, P.M.; Cui, M.; Grima, J.C.; Fan, R.Z.; De Mesy-Bentley, K.L.; Chen, L.; Zhuang, X.; Bowers, W.J.; Tieu, K. Drp1 Inhibition Attenuates Neurotoxicity and Dopamine Release Deficits in Vivo. Nat. Commun. 2014, 5, 5244.
- Zhao, F.; Austria, Q.; Wang, W.; Zhu, X. Mfn2 Overexpression Attenuates Mptp Neurotoxicity in Vivo. Int. J. Mol. Sci. 2021, 22, 601.
- Martín-Maestro, P.; Gargini, R.; García, E.; Simón, D.; Avila, J.; García-Escudero, V. Mitophagy Failure in APP and Tau Overexpression Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 70, 523–538.
- Martin, I.; Dawson, V.L.; Dawson, T.M. Recent Advances in the Genetics of Parkinson’s Disease. Annu. Rev. Genomics Hum. Genet. 2011, 12, 301–325.
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.K.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science 2004, 304, 1158–1160.
- Li, W.; Fu, Y.H.; Halliday, G.M.; Sue, C.M. PARK Genes Link Mitochondrial Dysfunction and Alpha-Synuclein Pathology in Sporadic Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 612476.
- Liu, X.; Liu, W.; Wang, C.; Chen, Y.; Liu, P.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Silibinin Attenuates Motor Dysfunction in a Mouse Model of Parkinson’s Disease by Suppression of Oxidative Stress and Neuroinflammation along with Promotion of Mitophagy. Physiol. Behav. 2021, 239, 113510.
- Dagda, R.K.; Cherra, S.J.; Kulich, S.M.; Tandon, A.; Park, D.; Chu, C.T. Loss of PINK1 Function Promotes Mitophagy through Effects on Oxidative Stress and Mitochondrial Fission. J. Biol. Chem. 2009, 284, 13843–13855.
- Morales, I.; Sanchez, A.; Puertas-Avendaño, R.; Rodriguez-Sabate, C.; Perez-Barreto, A.; Rodriguez, M. Neuroglial Transmitophagy and Parkinson’s Disease. Glia 2020, 68, 2277–2299.
- Choong, C.J.; Okuno, T.; Ikenaka, K.; Baba, K.; Hayakawa, H.; Koike, M.; Yokota, M.; Doi, J.; Kakuda, K.; Takeuchi, T.; et al. Alternative Mitochondrial Quality Control Mediated by Extracellular Release. Autophagy 2021, 17, 2962–2974.
- Araujo, B.G.; Souza e Silva, L.F.; de Barros Torresi, J.L.; Siena, A.; Valerio, B.C.O.; Brito, M.D.; Rosenstock, T.R. Decreased Mitochondrial Function, Biogenesis, and Degradation in Peripheral Blood Mononuclear Cells from Amyotrophic Lateral Sclerosis Patients as a Potential Tool for Biomarker Research. Mol. Neurobiol. 2020, 57, 5084–5102.
- Drabik, K.; Malińska, D.; Piecyk, K.; Dębska-Vielhaber, G.; Vielhaber, S.; Duszyński, J.; Szczepanowska, J. Effect of Chronic Stress Present in Fibroblasts Derived from Patients with a Sporadic Form of AD on Mitochondrial Function and Mitochondrial Turnover. Antioxidants 2021, 10, 938.
- Bennett, J.P.; Keeney, P.M. Alzheimer’s and Parkinson’s Brain Tissues Have Reduced Expression of Genes for MtDNA OXPHOS Proteins, Mitobiogenesis Regulator PGC-1α Protein and MtRNA Stabilizing Protein LRPPRC (LRP130). Mitochondrion 2020, 53, 154–157.
- Witte, M.E.; Nijland, P.G.; Drexhage, J.A.R.; Gerritsen, W.; Geerts, D.; Van Het Hof, B.; Reijerkerk, A.; De Vries, H.E.; Van Der Valk, P.; Van Horssen, J. Reduced Expression of PGC-1α Partly Underlies Mitochondrial Changes and Correlates with Neuronal Loss in Multiple Sclerosis Cortex. Acta Neuropathol. 2013, 125, 231–243.
- Terni, B.; Boada, J.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Mitochondrial ATP-Synthase in the Entorhinal Cortex Is a Target of Oxidative Stress at Stages I/II of Alzheimer’s Disease Pathology. Brain Pathol. 2010, 20, 222–233.
- Swerdlow, R.H. Mitochondria and Cell Bioenergetics: Increasingly Recognized Components and a Possible Etiologic Cause of Alzheimer’s Disease. Antioxidants Redox Signal. 2012, 16, 1434–1455.
- Albrecht, F.; Ballarini, T.; Neumann, J.; Schroeter, M.L. FDG-PET Hypometabolism Is More Sensitive than MRI Atrophy in Parkinson’s Disease: A Whole-Brain Multimodal Imaging Meta-Analysis. NeuroImage Clin. 2019, 21, 101594.
- Liot, G.; Valette, J.; Pépin, J.; Flament, J.; Brouillet, E. Energy Defects in Huntington’s Disease: Why “in Vivo” Evidence Matters. Biochem. Biophys. Res. Commun. 2017, 483, 1084–1095.
- Tondo, G.; Iaccarino, L.; Caminiti, S.P.; Presotto, L.; Santangelo, R.; Iannaccone, S.; Magnani, G.; Perani, D. The Combined Effects of Microglia Activation and Brain Glucose Hypometabolism in Early-Onset Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020, 12, 50.
- Johnson, E.C.B.; Dammer, E.B.; Duong, D.M.; Ping, L.; Zhou, M.; Yin, L.; Higginbotham, L.A.; Guajardo, A.; White, B.; Troncoso, J.C.; et al. Large-Scale Proteomic Analysis of Alzheimer’s Disease Brain and Cerebrospinal Fluid Reveals Early Changes in Energy Metabolism Associated with Microglia and Astrocyte Activation. Nat. Med. 2020, 26, 769–780.
- Tefera, T.W.; Steyn, F.J.; Ngo, S.T.; Borges, K. CNS Glucose Metabolism in Amyotrophic Lateral Sclerosis: A Therapeutic Target? Cell Biosci. 2021, 11, 14.
- Chu, J.S.; Liu, T.H.; Wang, K.L.; Han, C.L.; Liu, Y.P.; Michitomo, S.; Zhang, J.G.; Fang, T.; Meng, F.G. The Metabolic Activity of Caudate and Prefrontal Cortex Negatively Correlates with the Severity of Idiopathic Parkinson’s Disease. Aging Dis. 2019, 10, 847–853.
- Matthews, D.C.; Lerman, H.; Lukic, A.; Andrews, R.D.; Mirelman, A.; Wernick, M.N.; Giladi, N.; Strother, S.C.; Evans, K.C.; Cedarbaum, J.M.; et al. FDG PET Parkinson’s Disease-Related Pattern as a Biomarker for Clinical Trials in Early Stage Disease. NeuroImage Clin. 2018, 20, 572–579.
- Zhao, W.; De Felice, F.G.; Fernandez, S.; Chen, H.; Lambert, M.P.; Quon, M.J.; Krafft, G.A.; Klein, W.L. Amyloid Beta Oligomers Induce Impairment of Neuronal Insulin Receptors. FASEB J. 2008, 22, 246–260.
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s Disease a Type 3 Diabetes? A Critical Appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089.
- Aghanoori, M.R.; Smith, D.R.; Roy Chowdhury, S.; Sabbir, M.G.; Calcutt, N.A.; Fernyhough, P. Insulin Prevents Aberrant Mitochondrial Phenotype in Sensory Neurons of Type 1 Diabetic Rats. Exp. Neurol. 2017, 297, 148–157.
- Hong, C.T.; Chen, K.Y.; Wang, W.; Chiu, J.Y.; Wu, D.; Chao, T.Y.; Hu, C.J.; Chau, K.Y.D.; Bamodu, O.A. Insulin Resistance Promotes Parkinson’s Disease through Aberrant Expression of α-Synuclein, Mitochondrial Dysfunction, and Deregulation of the Polo-Like Kinase 2 Signaling. Cells 2020, 9, 740.
- Sintini, I.; Schwarz, C.G.; Martin, P.R.; Graff-Radford, J.; Machulda, M.M.; Senjem, M.L.; Reid, R.I.; Spychalla, A.J.; Drubach, D.A.; Lowe, V.J.; et al. Regional Multimodal Relationships between Tau, Hypometabolism, Atrophy, and Fractional Anisotropy in Atypical Alzheimer’s Disease. Hum. Brain Mapp. 2019, 40, 1618–1631.
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain Energy Rescue: An Emerging Therapeutic Concept for Neurodegenerative Disorders of Ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633.
- Ryu, J.C.; Zimmer, E.R.; Rosa-Neto, P.; Yoon, S.O. Consequences of Metabolic Disruption in Alzheimer’s Disease Pathology. Neurotherapeutics 2019, 16, 600–610.
- Dutta, R.; McDonough, J.; Yin, X.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial Dysfunction as a Cause of Axonal Degeneration in Multiple Sclerosis Patients. Ann. Neurol. 2006, 59, 478–489.
- Schattling, B.; Engler, J.B.; Volkmann, C.; Rothammer, N.; Woo, M.S.; Petersen, M.; Winkler, I.; Kaufmann, M.; Rosenkranz, S.C.; Fejtova, A.; et al. Bassoon Proteinopathy Drives Neurodegeneration in Multiple Sclerosis. Nat. Neurosci. 2019, 22, 887–896.
- Hariharan, A.; Shetty, S.; Shirole, T.; Jagtap, A.G. Potential of Protease Inhibitor in 3-Nitropropionic Acid Induced Huntington’s Disease like Symptoms: Mitochondrial Dysfunction and Neurodegeneration. Neurotoxicology 2014, 45, 139–148.
- Kumar, P.; Kumar, A. Protective Effect of Hesperidin and Naringin against 3-Nitropropionic Acid Induced Huntington’s like Symptoms in Rats: Possible Role of Nitric Oxide. Behav. Brain Res. 2010, 206, 38–46.
- Maegawa, H.; Niwa, H. Generation of mitochondrial toxin rodent models of Parkinson’s disease using 6-OHDA, MPTP, and rotenone. In Experimental Models of Parkinson’s Disease, 1st ed.; Imai, Y., Ed.; Springer: New York, NY, USA, 2021; Volume 2322, pp. 95–110.
- Prasad, E.M.; Hung, S.Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Antioxidants 2020, 9, 1007.
- Teil, M.; Arotcarena, M.L.; Dehay, B. A New Rise of Non-Human Primate Models of Synucleinopathies. Biomedicines 2021, 9, 272.
- Blesa, J.; Trigo-Damas, I.; del Rey, N.L.G.; Obeso, J.A. The Use of Nonhuman Primate Models to Understand Processes in Parkinson’s Disease. J. Neural Transm. 2018, 125, 325–335.
- Ravera, S.; Torazza, C.; Bonifacino, T.; Provenzano, F.; Rebosio, C.; Milanese, M.; Usai, C.; Panfoli, I.; Bonanno, G. Altered Glucose Catabolism in the Presynaptic and Perisynaptic Compartments of SOD1G93A Mouse Spinal Cord and Motor Cortex Indicates That Mitochondria Are the Site of Bioenergetic Imbalance in ALS. J. Neurochem. 2019, 151, 336–350.
- Li, W.; Feng, J.; Gao, C.; Wu, M.; Du, Q.; Tsoi, B.; Wang, Q.; Yang, D.; Shen, J. Nitration of Drp1 Provokes Mitophagy Activation Mediating Neuronal Injury in Experimental Autoimmune Encephalomyelitis. Free Radic. Biol. Med. 2019, 143, 70–83.
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. β-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science 2009, 324, 102–105.
This entry is offline, you can click here to edit this entry!
