In our unique transdisciplinary approach, we studied the fundamentals of blood pressure and examined its measuring modalities while focusing on their clinical use and sensing principles to identify material functionalities. Then, we carefully reviewed various categories of functional materials utilized in sensor building blocks allowing for comparative analysis of the performance of a wide range of materials throughout the sensor operational-life cycle. Not only this provides essential data to enhance the materials’ properties and optimize their performance, but also, it highlights new perspectives and provides suggestions to develop the next generation pressure sensors for clinical use.
- Blood Pressure Sensors
- wearable sensors
- sensing materials
- smart health monitor devices
- sensor operational lifecycle
Advancements in materials science and fabrication techniques have contributed to the significant growing attention to a wide variety of sensors for digital healthcare. While the progress in this area is tremendously impressive, few wearable sensors with the capability of real-time blood pressure monitoring are approved for clinical use. One of the key obstacles in the further development of wearable sensors for medical applications is the lack of comprehensive technical evaluation of sensor materials against the expected clinical performance. Here, we present an extensive review and critical analysis of various blood pressure approaches, sensing and transducing principles, as well as materials applied in the design and fabrication of wearable sensors.
1. Introduction
Cardiovascular diseases caused 31% of deaths worldwide [1][2][3], and recently, they had the highest confirmed death cases in Italy and China during the novel pandemic known as the coronavirus disease 2019 (COVID-19) [4][5]. In return, the demand for an accurate home-diagnostic tool for blood pressure measurements, along with other vital signs (e.g., temperature, respiratory rate) has increased massively. These tools, especially if enabled with telemedicine, will not only help assess a patient’s health status, triage the patient to appropriate care, determine potential diagnoses, and predict recovery, but also, it will help provide real-time medical monitoring, for instance, people in home-quarantine [6][7]. Hence, improving the precision and accuracy in blood pressure measurements can help significantly with early diagnosis and cardiovascular risk stratification [8][9][10][11], because inadequate performance in blood pressure measurement will increase current levels of fatal stroke and fatal myocardial infections [12], as well as impose an avoidable financial burden [13].
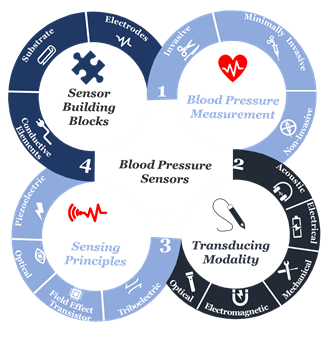
At the beginning of the twenty-first century, the use of sensors and mobile internet begins to provide a platform to continuously monitor all vital signs [14][15][16][17][18][19][20][21][22], including blood pressure. Not only does this help reduce the risk of cardiovascular complications, but also it supports making accurate and real-time healthcare data available for healthcare professionals at the office to assist select the best treatment strategies and consider the impact on patient outcomes [23][24][25][26][27][28]. Furthermore, this type of monitoring can save millions of lives around the globe annually [29][30][31]. Advancements in engineering and material science have been the main driver in the development of sensor technologies during the past decade[31][2][3]. Indeed, tactile sensors, and more precisely, skin-like soft electronics begin to transform healthcare [32][33][34]. In return, several studies highlight the crucial implications of this field and indicate that a timely review is necessary[35][36][37]. Since most studies focus on device functionality [38][39][40]., there is a need to investigate device clinical performance and capabilities beyond proof-of-concept measurements outside of the laboratory [41], following standardized evaluation approaches [42]. By precisely studying the unique nature of medical needs and evaluating the functionality of sensing principles and materials, we will comprehensively identify materials’ properties and their associated performance in line with structure strategies needed for accurate and continuous blood pressure measurement. Also, we will identify challenges along with future research opportunities. We aim to create a crosslink between healthcare practice and material science following a transdisciplinary approach illustrated in (Figure 1) to emphasize the importance of design and fabrication elements that have been either overlooked or compromised.
Figure 1. The transdisciplinary approach for the comprehensive review of the recent development in biomaterials used for an accurate yet continuous blood pressure measurement.
2. Blood Pressure Measurement
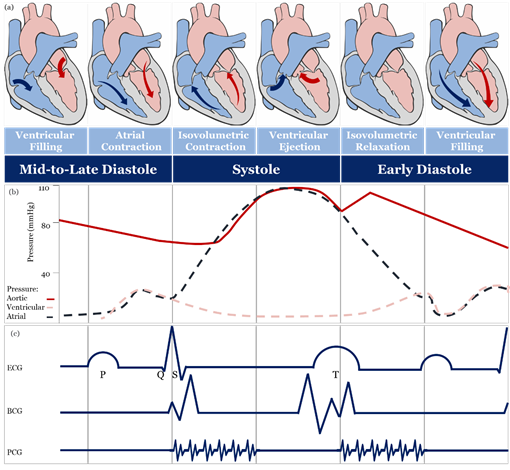
The theoretical and practical framework behind accurate blood pressure measurement is complex and, sometimes, overlooked entirely [43], therefore, understanding the effect of different approaches for blood pressure measurement is essential for developing accurate sensing materials suitable for medical use. The volume of blood ejected by the heart into the arteries, the elastance or stiffness of the walls of the arteries, and the rate at which the blood flows out of the arteries altogether affect blood pressure measurement [44]. During the cardiac cycle (Figure 2a,b), systolic pressure occurs as blood is ejected out of the heart and into the arteries, and diastolic pressure is created when the heart rests between heartbeats [43][44][45].
Figure 2. Schematic diagrams: (a) cardiac cycle, (b) arterial blood pressure versus ventricular and atrial blood pressure values, (c) morphological shapes of different signals associated with blood pressure. [46][47][48][49][50][51]
2.1. Invasive and Minimally Invasive Blood Pressure Measurement and Materials
Invasive blood pressure is directly measured by an intravascular catheter unit, which comprises of three main components: an intra-arterial cannula, an infusion tube, and a transducer [52][53]. The invasive approach is accurate and free of operator bias. Indeed, it is considered the gold standard for all other measures [54][55].Minimally-invasive blood pressure measurement is based on nonvascular implantable miniaturized sensors that are compatible with body tissues, and these devices can provide real-time monitoring of the cardiac cycle [56], including intravascular [57], intraocular [58] and intracranial [59][60][61]. The accuracy of a minimally invasive approach, in contrast to the invasive, is still controversial, and it may be due to the drift in sensitivity over a long time that affects long-term accuracy [62].
2.2. Non-Invasive Blood Pressure Measurement and Materials
2.2.1. Full Occlusion
The full-occlusion technique includes auscultatory [63], oscillometry [64], and palpatory [65]. Auscultatory and oscillometry are comparable to a gold standard [66], unlike palpatory, which is not used because obtaining a diastolic blood pressure measurement is difficult and may lead to considerable error. The accuracy of the oscillometry method can be highly affected by muscle contraction, noise artifacts, artery stiffness, age, and physical health , hence, validation and recalibration are crucial. Auscultatory and oscillometry methods are intermittent [67] and different cuff types [68][69][70][71][72][73] and fabrics [73][74][75][76][77] may lead to different blood pressure measurements [78][79].
2.2.2. Semi Occlusion
Semi-occlusion technique includes applanation tonometry [80][81][82][83], originally applied for monitoring intraocular blood pressure in glaucoma patients [84] using a Goldmann Applanation Tonometer [85] and quite recently contact lens-based sensors [86][87][88], and extended to include blood pressure measurement of the radial artery based on anisotropic conductive film[89] or a silicon-based MEMS sensing chip [90]. The accuracy of applanation tonometry is controversial, as it is highly dependent on artery location and changes in contact force required to maintain artery in an applanated status over time [91][92]. The volume clamp method of Peńăz, also known as vascular unloading, is a continuous blood pressure measurement [93], in which volumetric change in blood flow in a finger during the cardiac cycle is kept unchanged using a high-speed servo pump connected to a finger cuff and checked by a finger mounted photoplethysmography (PPG) sensor [94][95][96][97][98][99][100][101]. Several clinical studies demonstrated the accuracy and reproducibility of volume clamp methods [94], however, their accuracy is still controversial because different finger-cuff types and fabrics may lead to different blood pressure readings. The broad assumptions behind the use of the PPG sensor [95] and the underestimation of the effect of the significant difference in hydrostatic blood pressure between the finger and the heart may lead to an increase in inaccuracy [102][103]. Besides, the volume clamp method requires recalibration at regular times leading to an overestimation of systolic pressure [104]. It is recognized that the finger-cuff can be uncomfortable for patients, especially patients suffering from edema or patients with impaired peripheral blood flow [105][106].
2.2.3. No Occlusion
No-occlusion blood pressure measurement includes blood flow, pulse wave, and stroke volume methods. In the blood flow method, blood pressure is estimated utilizing the bifurcated or diseased artery geometry and the pulsatile blood flow equations [107][108][109]. The pulse wave method is a simplified form of pulsatile blood flow equations under certain assumptions is used [110]. In the stroke volume method, mean arterial blood pressure is estimated through measuring changes in the volume of blood pumped from the left ventricle (i.e., cardiac output) and the resistance that must be overcome to push blood and create flow in arteries (i.e., systemic vascular resistance) or through estimating cardiac output from O2 consumption levels [111]. The accuracy of the contact [112][113] and non-contact [114] sensing modalities of the blood flow method is controversial. Contact sensing has met the gold standard level of accuracy under certain conditions and failed to meet it under others, whereas non-contact sensing modalities show a significant reduction in diagnostic performance. Likewise, a non-invasive form of FFR (i.e., FFRCT) has been described, with some studies showing that it is safe and feasible [115] and with others showing that current clinical trial data are insufficient to make a recommendation for its use in clinical practice [116][117][118].
Sensors based on stroke volume methods, including wearable ICG/ECG, are widely used [119]. The wearable ICG/ECG includes flexible dry electrodes made of a Ti-Au composite [120], a Ni-P plated polyester fabric [121], Ag flakes with MWCNT/PDMS composite [122], a woven fabric treated with PEDOT:PSS [123] and an Ecoflex-Ag MPs self-adhesive micropillar electrode inspired by gecko and grasshopper feet [124]. Furthermore, they can be fabricated of an EPDM rubber electrode containing various additives such as carbon, stainless steel fibers, and CNT [125].
Wearable ICG/ECG performance depends on the design and fabrication of high sensitivity electrodes and the continuous contact with skin, as well as their location when placed on the human body surface . Also, their accuracy is mainly associated with the level of calculation complexity, which requires many mathematical assumptions, as well as measurement and physiological artifacts [126].
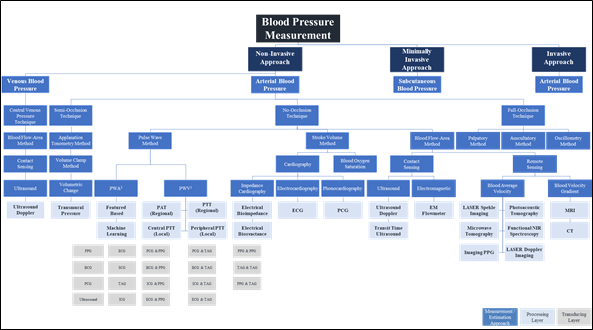
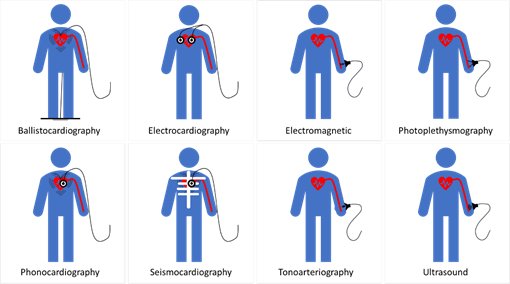
The pulse wave method is widely used in wearable and wireless applications due to its ability to integrate with a wide variety of transducers used in sensor application architectures. In addition to the effect of changes in measurement and physiological artifacts [127] and the pulse wave method does not collectively consider the impact of changes in physiological factors in blood viscosity, vascular wall elasticity, peripheral resistance of the arterial tree, and morphological characteristics in pressure pulse wave that vary regularly [128]. Figure 4 depicts the landscape of blood pressure measurement, approaches, methods, processing, and transducing modality layers, and Table 1 summarizes our analysis findings and highlights areas for further investigation.
Figure 3. Blood pressure measurement landscape; approaches, methods, processing, and transducing layers. 1 PWA: At least a single pulsatility sensor or a single cardiovascular sensor is implemented; 2 PWV: At least two pulsatility sensors and/or additional cardiovascular sensor is implemented.
Non-invasive methods—with no-occlusion blood pressure measurements based on wearable devices—offer a promising future. Failing to choose the right materials for the fabrication of wearable devices can lead to either high noise in the received signal, which affects accuracy, or red and itchy rash in the skin caused by direct contact of the materials or even an allergic reaction to a body part causing highly frequent diseases that are clinically referred to by contact dermatitis [129]. Long direct contact of skin with medically unsuitable wearable device materials can foster an attractive and supportive environment for harmful microbiota, increasing the risk of infectious skin diseases, especially amongst patients with chronic diseases [130][131][.
3. Sensing Principles
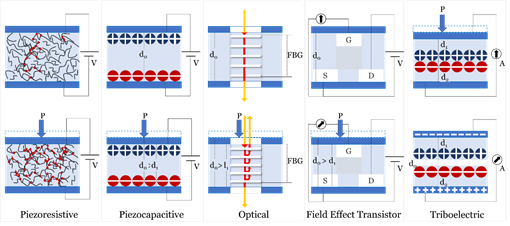
Sensing principles used in blood pressure sensors include; Piezoelectric, Piezocapacitive, Optical, Field Effect Transistor (FET), and Triboelectric. Figure 4 illustrates the mechanism of each of the fundamental sensing principles studied in this review.
Figure 4. Fundamental sensing principles used in sensors
4.Sensor Building Blocks
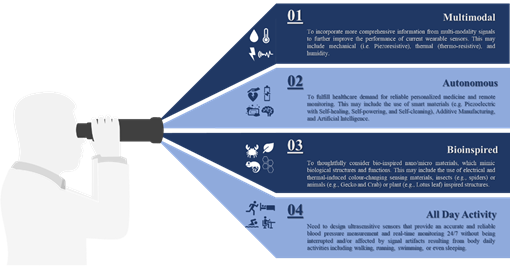
5.Operational Lifecycle
6.Outlook
Biomaterials have attracted considerable attention due to their exceptional performance and are, therefore, well-received as one of the future building blocks of digital healthcare. For blood pressure measurements and real-time monitoring, wearable sensors have not comprehensively reached a medically accepted level of functionality to replace intermittent cuff-based devices. This gap has been seemingly associated with a disconnect between the two knowledge areas, particularly, blood pressure measurement approaches and their related modalities at one side, and functional materials design and fabrication strategies and their relevant sensing mechanisms at the other one.
Invasive and minimally invasive blood pressure measurement does not support continuous measurement and real-time monitoring. Hence, ECG and PPG signals are mostly used to monitor health status without disturbing the users during daily activities. It would be beneficial to incorporate more comprehensive information collected from multi-modality signals such as mechanical (i.e., piezoresistive), thermal (i.e., thermoresistive), or even humidity to further improve the performance of current wearable sensors rather than considering a single sensing principle as illustrated in this review.
Apart from the rapid development demonstrated in this review, there is a bright future for tactile sensors utilizing smart materials (e.g., piezoelectric, self-healing, self-powering, self-cleaning), additive manufacturing, big data analytics (e.g., artificial intelligence) and cloud computing to fulfill healthcare demand for personalized medicine and remote monitoring. We found that sensitivity was the focus for most of the developed devices and those with piezoresistive mechanisms generally showed high performance when compared with others [242,259,271,283,293]. Although piezocapacitive-based sensors still show excellent detectability and sensitivity, they are more susceptible to noise resulting from field interaction and fringing capacitance, as well as, other factors such as temperature . FET-based sensors show high sensitivity with excellent response time due to their perfect functionalities of signal transduction and amplification, but their flexibility measured under continuous cycles of loading/unloading, is still a challenge while the best performing device of this category shows a tiny bending radius lower than 0.02 cm with no significant variation in its electrical characteristics after more than 200 cycles a piezoresistive-based sensor with a similar sensitivity can show consistent performance under continuous cycles of loading/unloading on the order of thousands. Also, FET based sensors require relatively higher operating voltage when compared to that of piezoresistive. This variation in performance should be considered as a motive, not only for further development of existing sensing principles but also for further investigation of novel mechanisms to go beyond meeting high sensitivity requirements to include linear performance without hysteresis.
The high sensitivity requirements have been achieved through the effective utilization of newly developed functional materials, such as PVDF/rGO or ultrathin NWs, the optimization of device geometry, such as the design inspired by insects’ sensing capabilities or plant leaves’ morphology or the microstructure of human skin. Other strategies include the reduction of active materials concentration in nanocomposite matrix through alignment , the utilization of novel additives manufacturing techniques such as LIG, or the imprinting technique to control the patterns and orientation of functional materials by template restriction . The design and fabrication of wearable sensors with autonomous capabilities include wireless transmission self-powered , self-healed and self-degraded will certainly enable continuous diagnosis of cardiovascular disease. However, these features and similar ones are no longer considered desirable, but they are becoming as crucial as other performance measures since they will allow automatic reparation of device malfunctions and disposal. Furthermore, the integrated sensing modality of tactile, temperature, and humidit to extract additional features from other signals (e.g., respiratory) with accuracy under the interface of strong body movement in real-time, will boost the performance of pressure measurements.
The performance of wearable sensors, capitalizing on the recent advancement in machine learning and cloud computation, can be further boosted by selecting optimal features that can contribute to dynamic blood pressure changes. In return, this will provide an accurate blood pressure measurement noninvasively and continuously, help enable early prevention and personalized treatment of hypertension, and reduce its burden on society. Finally, there is an essential need for a multidisciplinary approach encompasses of different knowledge areas, mainly, data, material, medical, and engineering sciences to ensure seamless integration between various sensor components and architecture, and address other challenges associated with sensor overall performance, as well as, evaluate the impact of each on device reliability and efficiency. Figure 6 summarises future development in blood pressure measurement and real-time monitoring.
Figure 7. Summary of future development in the field of blood pressure measurement and real-time monitoring.
This entry is adapted from the peer-reviewed paper 10.3390/s20164484
References
- World Health Organization Cardiovascular Diseases (CVDs) Report; World Health Organization (WHO): 2019.
- Seminara, L.; Pinna, L.; Ibrahim, A.; Noli, L.; Capurro, M.; Caviglia, S.; Gastaldo, P.; Valle, M., Electronic Skin: Achievements, Issues and Trends. Procedia Technology 2014, 15, 549-558.
- Shimonomura, K., Tactile Image Sensors Employing Camera: A Review. Sensors (Basel) 2019, 19, (18), 3933.
- COVID-19 Surveillance Group Characteristics of COVID-19 patients dying in Italy Report based on available data on March 24th, 2020; The Italian National Health Service Italy, 27/03/2020, 2020.
- World Health Organization (WHO)-China Joint Mission Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) World Health Organization (WHO): 28/02/2020, 2020.
- Kovacs, R. J.; Moyer, D. V., Statement on the need for increased access to telehealth to combat community spread of COVID-19. In American College of Cardiology and American College of Physicians,: Washington D.C. , 2020; p 1.
- Perl, T. M.; Price, C. S., Managing Emerging Infectious Diseases: Should Travel Be the Fifth Vital Sign? Ann Intern Med 2020.
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; Galderisi, M.; Grobbee, D. E.; Jaarsma, T.; Kirchhof, P.; Kjeldsen, S. E.; Laurent, S.; Manolis, A. J.; Nilsson, P. M.; Ruilope, L. M.; Schmieder, R. E.; Sirnes, P. A.; Sleight, P.; Viigimaa, M.; Waeber, B.; Zannad, F.; Task Force, M., 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013, 31, (7), 1281-357.
- Bentzon, J. F.; Otsuka, F.; Virmani, R.; Falk, E., Mechanisms of plaque formation and rupture. Circ Res 2014, 114, (12), 1852-66.
- RenJi, H. Early Detection of Cardiac Impairment and Prediction of RV Hypertrophy in Patients With CTD. https://ClinicalTrials.gov/show/NCT04297371
- Sierra, C.; de la Sierra, A., Early detection and management of the high-risk patient with elevated blood pressure. Vasc Health Risk Manag 2008, 4, (2), 289-96.
- Handler, J., The importance of accurate blood pressure measurement. Perm J 2009, 13, (3), 51-4.
- Poncette, A. S.; Spies, C.; Mosch, L.; Schieler, M.; Weber-Carstens, S.; Krampe, H.; Balzer, F., Clinical Requirements of Future Patient Monitoring in the Intensive Care Unit: Qualitative Study. JMIR Med Inform 2019, 7, (2), e13064.
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H., Point-of-care testing based on smartphone: The current state-of-the-art (2017-2018). Biosens Bioelectron 2019, 132, 17-37.
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; Meng, K.; Lin, Z.; Uy, C.; Yang, J.; Chen, J., Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens Bioelectron 2020, 155, 112064.
- Lisi, F.; Peterson, J. R.; Gooding, J. J., The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens Bioelectron 2020, 148, 111835.
- Shandilya, R.; Bhargava, A.; Bunkar, N.; Tiwari, R.; Goryacheva, I. Y.; Mishra, P. K., Nanobiosensors: Point-of-care approaches for cancer diagnostics. Biosens Bioelectron 2019, 130, 147-165.
- Xu, D.; Huang, X.; Guo, J.; Ma, X., Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens Bioelectron 2018, 110, 78-88.
- Escobedo, P.; Erenas, M. M.; Martinez-Olmos, A.; Carvajal, M. A.; Gonzalez-Chocano, S.; Capitan-Vallvey, L. F.; Palma, A. J., General-purpose passive wireless point-of-care platform based on smartphone. Biosens Bioelectron 2019, 141, 111360.
- Wang, T.; Mei, Q.; Tao, Z.; Wu, H.; Zhao, M.; Wang, S.; Liu, Y., A smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens Bioelectron 2020, 148, 111791.
- Kurbanoglu, S.; Ozkan, S. A.; Merkoci, A., Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens Bioelectron 2017, 89, (Pt 2), 886-898.
- Song, Y.; Min, J.; Gao, W., Wearable and Implantable Electronics: Moving toward Precision Therapy. ACS Nano 2019, 13, (11), 12280-12286.
- Iftikhar, Z.; Lahdenoja, O.; Jafari Tadi, M.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M., Multiclass Classifier based Cardiovascular Condition Detection Using Smartphone Mechanocardiography. Scientific reports 2018, 8, (1), 9344.
- Pfeiffer, S., The Vision of “Industrie 4.0” in the Making-a Case of Future Told, Tamed, and Traded. Nanoethics 2017, 11, (1), 107-121.
- Schwab K., The fourth industrial revolution. World Economic, Forum: 2016.
- Topol, E. J., High-performance medicine: the convergence of human and artificial intelligence. Nat Med 2019, 25, (1), 44-56.
- Chrysant, S. G., A new paradigm in the treatment of the cardiovascular disease continuum: focus on prevention. Hippokratia 2011, 15, (1), 7-11.
- Engel, J.; van der Wulp, I.; Poldervaart, J. M.; Reitsma, J. B.; de Bruijne, M. C.; Wagner, C., Clinical decision-making of cardiologists regarding admission and treatment of patients with suspected unstable angina or non-ST-elevation myocardial infarction: protocol of a clinical vignette study. BMJ Open 2015, 5, (4), e006441.
- Karunathilake, S. P.; Ganegoda, G. U., Secondary Prevention of Cardiovascular Diseases and Application of Technology for Early Diagnosis. Biomed Res Int 2018, 2018, 5767864.
- McGill, H. C., Jr.; McMahan, C. A.; Gidding, S. S., Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation 2008, 117, (9), 1216-27.
- D’Addona, D. M.; Rongo, R.; Teti, R.; Martina, R., Bio-compatible cyber-physical system for cloud-based customizable sensor monitoring of pressure conditions. Procedia CIRP 2018, 67, 150-155.
- Xu, S.; Jayaraman, A.; Rogers, J. A., Skin sensors are the future of health care. Nature 2019, 571, (7765), 319-321.
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S. W.; Gaitonde, S.; Begtrup, G.; Katchman, B. A., Accessing analytes in biofluids for peripheral biochemical monitoring. Nat Biotechnol 2019, 37, (4), 407-419.
- Chung, H. U.; Kim, B. H.; Lee, J. Y.; Lee, J.; Xie, Z.; Ibler, E. M.; Lee, K.; Banks, A.; Jeong, J. Y.; Kim, J.; Ogle, C.; Grande, D.; Yu, Y.; Jang, H.; Assem, P.; Ryu, D.; Kwak, J. W.; Namkoong, M.; Park, J. B.; Lee, Y.; Kim, D. H.; Ryu, A.; Jeong, J.; You, K.; Ji, B.; Liu, Z.; Huo, Q.; Feng, X.; Deng, Y.; Xu, Y.; Jang, K. I.; Kim, J.; Zhang, Y.; Ghaffari, R.; Rand, C. M.; Schau, M.; Hamvas, A.; Weese-Mayer, D. E.; Huang, Y.; Lee, S. M.; Lee, C. H.; Shanbhag, N. R.; Paller, A. S.; Xu, S.; Rogers, J. A., Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363, (6430), eaau0780.
- Yao, S.; Swetha, P.; Zhu, Y., Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv Healthc Mater 2018, 7, (1), 1700889.
- Ray, T. R.; Choi, J.; Bandodkar, A. J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J. A., Bio-Integrated Wearable Systems: A Comprehensive Review. Chemical Reviews 2019, 119, (8), 5461-5533.
- Yetisen, A. K.; Martinez-Hurtado, J. L.; Unal, B.; Khademhosseini, A.; Butt, H., Wearables in Medicine. Adv Mater 2018, 30, (33), e1706910.
- Dooley, E. E.; Golaszewski, N. M.; Bartholomew, J. B., Estimating Accuracy at Exercise Intensities: A Comparative Study of Self-Monitoring Heart Rate and Physical Activity Wearable Devices. JMIR Mhealth Uhealth 2017, 5, (3), e34.
- Izmailova, E. S.; Wagner, J. A.; Perakslis, E. D., Wearable Devices in Clinical Trials: Hype and Hypothesis. Clin Pharmacol Ther 2018, 104, (1), 42-52.
- Bent, B.; Goldstein, B. A.; Kibbe, W. A.; Dunn, J. P., Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit Med 2020, 3, 18.
- Kaisti, M.; Panula, T.; Leppanen, J.; Punkkinen, R.; Jafari Tadi, M.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, J.; Kostiainen, P.; Meriheina, U.; Koivisto, T.; Pankaala, M., Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. NPJ Digit Med 2019, 2, (1), 39.
- Duking, P.; Fuss, F. K.; Holmberg, H. C.; Sperlich, B., Recommendations for Assessment of the Reliability, Sensitivity, and Validity of Data Provided by Wearable Sensors Designed for Monitoring Physical Activity. JMIR Mhealth Uhealth 2018, 6, (4), e102.
- Magder, S., The meaning of blood pressure. Crit Care 2018, 22, (1), 257.
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W. W., McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. CRC Press: 2011.
- Kleinstreuer, C., Biofluid dynamics : principles and selected applications. CRC/Taylor & Francis: Boca Raton, FL, 2006.
- Belz, G. G., Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther 1995, 9, (1), 73-83.
- Ogedegbe, G.; Pickering, T., Principles and techniques of blood pressure measurement. Cardiol Clin 2010, 28, (4), 571-86.
- Kim, C. S.; Ober, S. L.; McMurtry, M. S.; Finegan, B. A.; Inan, O. T.; Mukkamala, R.; Hahn, J. O., Ballistocardiogram: Mechanism and Potential for Unobtrusive Cardiovascular Health Monitoring. Sci Rep 2016, 6, 31297.
- Varghees, V. N.; Ramachandran, K. I., A novel heart sound activity detection framework for automated heart sound analysis. Biomedical Signal Processing and Control 2014, 13, 174-188.
- Chowdhury, M. H.; Cheung, R. C. C., Reconfigurable Architecture for Multi-lead ECG Signal Compression with High-frequency Noise Reduction. Sci Rep 2019, 9, (1), 17233.
- McEniery, C. M.; Cockcroft, J. R.; Roman, M. J.; Franklin, S. S.; Wilkinson, I. B., Central blood pressure: current evidence and clinical importance. Eur Heart J 2014, 35, (26), 1719-25.
- Romagnoli, S.; Ricci, Z.; Quattrone, D.; Tofani, L.; Tujjar, O.; Villa, G.; Romano, S. M.; De Gaudio, A. R., Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care 2014, 18, (6), 644.
- Crystal, G. J.; Assaad, S. I.; Heerdt, P. M., Cardiovascular Physiology. In Pharmacology and Physiology for Anesthesia, Hemmings, H. C.; Egan, T. D., Eds. Elsevier: Philadelphia, 2019; pp 473-519.
- Versi, E., “Gold standard” is an appropriate term. BMJ 1992, 305, (6846), 187.
- Pour-Ghaz, I.; Manolukas, T.; Foray, N.; Raja, J.; Rawal, A.; Ibebuogu, U. N.; Khouzam, R. N., Accuracy of non-invasive and minimally invasive hemodynamic monitoring: where do we stand? Ann Transl Med 2019, 7, (17), 421.
- Murphy, O. H.; Bahmanyar, M. R.; Borghi, A.; McLeod, C. N.; Navaratnarajah, M.; Yacoub, M. H.; Toumazou, C., Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor. Biomed Microdevices 2013, 15, (5), 737-49.
- Melki, S.; Todani, A.; Cherfan, G., An implantable intraocular pressure transducer: initial safety outcomes. JAMA Ophthalmol 2014, 132, (10), 1221-5.
- Kawoos, U.; McCarron, R. M.; Auker, C. R.; Chavko, M., Advances in Intracranial Pressure Monitoring and Its Significance in Managing Traumatic Brain Injury. Int J Mol Sci 2015, 16, (12), 28979-97.
- Chen, X.; Brox, D.; Assadsangabi, B.; Mohamed Ali, M. S.; Takahata, K., A stainless-steel-based implantable pressure sensor chip and its integration by microwelding. Sensors and Actuators A: Physical 2017, 257, 134-144.
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M. R.; Huang, Y.; Ray, W. Z.; Zhou, W.; Rogers, J. A., Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci Adv 2019, 5, (7), eaaw1899.
- Potkay, J. A., Long term, implantable blood pressure monitoring systems. Biomed Microdevices 2008, 10, (3), 379-92.
- Benmira, A.; Perez-Martin, A.; Schuster, I.; Aichoun, I.; Coudray, S.; Bereksi-Reguig, F.; Dauzat, M., From Korotkoff and Marey to automatic non-invasive oscillometric blood pressure measurement: does easiness come with reliability? Expert Rev Med Devices 2016, 13, (2), 179-89.
- R. Raamat, K. J., J. Talts, and J. Kivastik In A model-based retrospective analysis of the fixed-ratio oscillometric blood pressure measurement, 13th IEEE International Conference on BioInformatics and BioEngineering, IEEE BIBE 2013, Chania, 2013; Chania, 2013.
- Dinesh, S.; M, B.; Charles, F.; Upendra, K.; Subramaniam, N.; Jamshed, D., Palpatory Method of Measuring Diastolic Blood Pressure. Journal of Anaesthesiology, Clinical Pharmacology 2010, 26.
- Odagiri, T.; Morita, T.; Yamauchi, T.; Imai, K.; Tei, Y.; Inoue, S., Convenient measurement of systolic pressure: the reliability and validity of manual radial pulse pressure measurement. J Palliat Med 2014, 17, (11), 1226-30.
- International Organization for Standardization, ISO 81060-2:2018 Non-invasive sphygmomanometers - Part 2: Clinical investigation of intermittent automated measurement type. In 2018; p 36.
- Ringrose, J. S.; McLean, D.; Ao, P.; Yousefi, F.; Sankaralingam, S.; Millay, J.; Padwal, R., Effect of Cuff Design on Auscultatory and Oscillometric Blood Pressure Measurements. Am J Hypertens 2016, 29, (9), 1063-9.
- Bilo, G.; Sala, O.; Perego, C.; Faini, A.; Gao, L.; Gluszewska, A.; Ochoa, J. E.; Pellegrini, D.; Lonati, L. M.; Parati, G., Impact of cuff positioning on blood pressure measurement accuracy: may a specially designed cuff make a difference? Hypertens Res 2017, 40, (6), 573-580.
- O’Brien, E., Review: a century of confusion; which bladder for accurate blood pressure measurement? J Hum Hypertens 1996, 10, (9), 565-72.
- Tochikubo, O.; Watanabe, J.; Hanada, K.; Miyajima, E.; Kimura, K., A new double cuff sphygmotonometer for accurate blood pressure measurement. Hypertens Res 2001, 24, (4), 353-7.
- Brown, M. A.; Buddle, M. L.; Whitworth, J. A., Measurement of blood pressure during pregnancy: evaluation of the ‘TriCUFF’. Aust N Z J Obstet Gynaecol 1993, 33, (1), 48-50.
- Bonso, E.; Saladini, F.; Zanier, A.; Benetti, E.; Dorigatti, F.; Palatini, P., Accuracy of a single rigid conical cuff with standard-size bladder coupled to an automatic oscillometric device over a wide range of arm circumferences. Hypertens Res 2010, 33, (11), 1186-91.
- Duffy M. K.; M., W. BLOOD PRESSURE CUFF AND TO A METHOD OF MAKING THE SAME 1992.
- Ledford J.; Drake R.; Ellenburg L.; Jarvis G.; L., P. E. BLADDERLESS BLOOD PRESSURE CUFF. 1998.
- Garrett R. J. DISPOSABLE MEDICAL PRESSURE CUFFS AND METHOD OF PRODUCTION. 1994.
- VivenzioIan R. L.; Edwards I. K.; Lia R. A.; Perkins J.; Karla S. R. RECYCLABLE OR BIODEGRADABLE BLOOD PRESSURE CUFF. 2014.
- Li, H.; Bao, H.; Bok, K. X.; Lee, C. Y.; Li, B.; Zin, M. T.; Kang, L., High durability and low toxicity antimicrobial coatings fabricated by quaternary ammonium silane copolymers. Biomater Sci 2016, 4, (2), 299-309.
- McCaughey E.; Higgins T.; Shlisky T. ANTIMICROBIAL BLOOD PRESSURE CUFF LINER. 2010.
- Deselle C.T; Durgag K.; Paul B.; Gunn V.; Pendleton B.; Provonchee R. BLOOD PRESSURE CUFF SHIELD INCORPORATING ANTIMICROBIAL TECHNOLOGY. 2014.
- De Smedt, S., Noninvasive intraocular pressure monitoring: current insights. Clin Ophthalmol 2015, 9, 1385-92.
- Drzewiecki, G.; Krishna, G.; Katta, H., Method of deflection corrected tonometry with phantom vessel experiments. Comput Biol Med 2019, 104, 329-334.
- Garcia-Ortiz, L.; Recio-Rodriguez, J. I.; Agudo-Conde, C.; Maderuelo-Fernandez, J. A.; Patino-Alonso, M. C.; de Cabo-Laso, A.; Rodriguez-Martin, C.; Gonzalez-Sanchez, J.; Rodriguez-Sanchez, E.; Gomez-Marcos, M. A.; on behalf the, E. V. A. I. g., Noninvasive validation of central and peripheral augmentation index estimated by a novel wrist-worn tonometer. J Hypertens 2018, 36, (11), 2204-2214.
- Hirano, H.; Fukuchi, T.; Kurita, Y.; Kandori, A.; Sano, Y.; Nakamura, R.; Saeki, N.; Kawamoto, M.; Yoshizumi, M.; Tsuji, T., Development of a Palpable Carotid Pulse Pressure Sensor Using Electromagnetic Induction. IEEJ Transactions on Electronics, Information and Systems 2012, 132, (12), 1934-1942.
- Okafor, K. C.; Brandt, J. D., Measuring intraocular pressure. Curr Opin Ophthalmol 2015, 26, (2), 103-9.
- Ozcura, F.; Yildirim, N.; Sahin, A.; Colak, E., Comparison of Goldmann applanation tonometry, rebound tonometry and dynamic contour tonometry in normal and glaucomatous eyes. Int J Ophthalmol 2015, 8, (2), 299-304.
- Leonardi, M.; Leuenberger, P.; Bertrand, D.; Bertsch, A.; Renaud, P. In A soft contact lens with a MEMS strain gage embedded for intraocular pressure monitoring, TRANSDUCERS ‘03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems. Digest of Technical Papers (Cat. No.03TH8664), 8-12 June 2003, 2003; 2003; pp 1043-1046 vol.2.
- Chen, G. Z.; Chan, I. S.; Leung, L. K.; Lam, D. C., Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med Eng Phys 2014, 36, (9), 1134-9.
- Zhang, Y.; Chen, Y.; Man, T.; Huang, D.; Li, X.; Zhu, H.; Li, Z., High resolution non-invasive intraocular pressure monitoring by use of graphene woven fabrics on contact lens. Microsyst Nanoeng 2019, 5, (1), 39.
- Lee, B.; Jeong, J.; Kim, J.; Kim, B.; Chun, K., Cantilever arrayed blood pressure sensor for arterial applanation tonometry. IET Nanobiotechnol 2014, 8, (1), 37-43.
- Roh, D.; Han, S.; Park, J.; Shin, H., Development of a Multi-Array Pressure Sensor Module for Radial Artery Pulse Wave Measurement. Sensors (Basel) 2019, 20, (1).
- Agnoletti, D.; Millasseau, S. C.; Topouchian, J.; Zhang, Y.; Safar, M. E.; Blacher, J., Pulse wave analysis with two tonometric devices: a comparison study. Physiol Meas 2014, 35, (9), 1837-48.
- Hansen, S.; Staber, M., Oscillometric blood pressure measurement used for calibration of the arterial tonometry method contributes significantly to error. Eur J Anaesthesiol 2006, 23, (9), 781-7.
- Uemura, K.; Kawada, T.; Sugimachi, M., A Novel Minimally Occlusive Cuff Method Utilizing Ultrasound Vascular Imaging for Stress-Free Blood Pressure Measurement: A-Proof-of-Concept Study. IEEE Trans Biomed Eng 2019, 66, (4), 934-945.
- Saugel, B.; Cecconi, M.; Hajjar, L. A., Noninvasive Cardiac Output Monitoring in Cardiothoracic Surgery Patients: Available Methods and Future Directions. J Cardiothorac Vasc Anesth 2019, 33, (6), 1742-1752.
- Saugel, B.; Dueck, R.; Wagner, J. Y., Measurement of blood pressure. Best Pract Res Clin Anaesthesiol 2014, 28, (4), 309-22.
- Gerdt D.W.; Adkins C.; Baruch M. HYDROSTATIC FINGER CUFF FOR BLOOD WAVE FORMANALYSIS AND DAGNOSTIC SUPPORT 2012.
- Cline R.L.; Rosthauser J.W.; Markusch P.H. REMOVABLE POLYURETHANE ADHESIVES WITH IMPROVED TEMPERATURE RESISTANCE PROPERTIES. 2000.
- Huber C.; Grüllenberger R.; Fortin J. DISPOSABLE AND DETACHABLE SENSOR FOR CONTINUOUS NON-INVASIVE ARTERIAL BLOOD PRESSURE MONITORING. 2011.
- Westerhof B.; Schraa O.; Van Groeningen C.J.E.; Li P. SELF CLOSING FINGER CUFF. 2018.
- Edwards Lifesciences, ClearSight System. In Innovation for Noninvasive Hemodynamic Management, Edwards Lifesciences, Ed. Edwards Lifesciences: 2018.
- Hertzman, A. B., The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am. J. Physiol. Cell Physiol. 1938, 124, 328-340.
- Ding, X. R.; Zhao, N.; Yang, G. Z.; Pettigrew, R. I.; Lo, B.; Miao, F.; Li, Y.; Liu, J.; Zhang, Y. T., Continuous Blood Pressure Measurement From Invasive to Unobtrusive: Celebration of 200th Birth Anniversary of Carl Ludwig. IEEE J Biomed Health Inform 2016, 20, (6), 1455-1465.
- Cluff, K.; Becker, R.; Jayakumar, B.; Han, K.; Condon, E.; Dudley, K.; Szatkowski, G.; Pipinos, II; Amick, R. Z.; Patterson, J., Passive Wearable Skin Patch Sensor Measures Limb Hemodynamics Based on Electromagnetic Resonance. IEEE Trans Biomed Eng 2018, 65, (4), 847-856.
- Birch, A. A.; Morris, S. L., Do the Finapres and Colin radial artery tonometer measure the same blood pressure changes following deflation of thigh cuffs? Physiological measurement 2003, 24, (3), 653-60.
- Hohn, A.; Defosse, J. M.; Becker, S.; Steffen, C.; Wappler, F.; Sakka, S. G., Non-invasive continuous arterial pressure monitoring with Nexfin does not sufficiently replace invasive measurements in critically ill patients. Br J Anaesth 2013, 111, (2), 178-84.
- Berkelmans, G. F. N.; Kuipers, S.; Westerhof, B. E.; Spoelstra-de Man, A. M. E.; Smulders, Y. M., Comparing volume-clamp method and intra-arterial blood pressure measurements in patients with atrial fibrillation admitted to the intensive or medium care unit. J Clin Monit Comput 2018, 32, (3), 439-446.
- Xiong, G.; Figueroa, C. A.; Xiao, N.; Taylor, C. A., Simulation of blood flow in deformable vessels using subject-specific geometry and spatially varying wall properties. Int J Numer Method Biomed Eng 2011, 27, (7), 1000-1016.
- Mukkamala, R.; Hahn, J. O.; Inan, O. T.; Mestha, L. K.; Kim, C. S.; Toreyin, H.; Kyal, S., Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans Biomed Eng 2015, 62, (8), 1879-901.
- Ding, X.; Zhang, Y. T., Pulse transit time technique for cuffless unobtrusive blood pressure measurement: from theory to algorithm. Biomed Eng Lett 2019, 9, (1), 37-52.
- Davies, J. I.; Struthers, A. D., Beyond blood pressure: pulse wave analysis--a better way of assessing cardiovascular risk? Future Cardiol 2005, 1, (1), 69-78.
- De Cort, S. C.; Innes, J. A.; Barstow, T. J.; Guz, A., Cardiac output, oxygen consumption and arteriovenous oxygen difference following a sudden rise in exercise level in humans. J Physiol 1991, 441, 501-12.
- Alruwaili, F.; Cluff, K.; Griffith, J.; Farhoud, H., Passive Self Resonant Skin Patch Sensor to Monitor Cardiac Intraventricular Stroke Volume Using Electromagnetic Properties of Blood. IEEE J Transl Eng Health Med 2018, 6, 1900709.
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; Bhaskaran, S.; Gu, Y.; Makihata, M.; Guo, Y.; Lei, Y.; Chen, Y.; Wang, C.; Li, Y.; Zhang, T.; Chen, Z.; Pisano, A. P.; Zhang, L.; Zhou, Q.; Xu, S., Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat Biomed Eng 2018, 2, (9), 687-695.
- Task Force, M.; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; Ferreira, J. R.; Gersh, B. J.; Gitt, A. K.; Hulot, J. S.; Marx, N.; Opie, L. H.; Pfisterer, M.; Prescott, E.; Ruschitzka, F.; Sabate, M.; Senior, R.; Taggart, D. P.; van der Wall, E. E.; Vrints, C. J.; Guidelines, E. S. C. C. f. P.; Zamorano, J. L.; Achenbach, S.; Baumgartner, H.; Bax, J. J.; Bueno, H.; Dean, V.; Deaton, C.; Erol, C.; Fagard, R.; Ferrari, R.; Hasdai, D.; Hoes, A. W.; Kirchhof, P.; Knuuti, J.; Kolh, P.; Lancellotti, P.; Linhart, A.; Nihoyannopoulos, P.; Piepoli, M. F.; Ponikowski, P.; Sirnes, P. A.; Tamargo, J. L.; Tendera, M.; Torbicki, A.; Wijns, W.; Windecker, S.; Document, R.; Knuuti, J.; Valgimigli, M.; Bueno, H.; Claeys, M. J.; Donner-Banzhoff, N.; Erol, C.; Frank, H.; Funck-Brentano, C.; Gaemperli, O.; Gonzalez-Juanatey, J. R.; Hamilos, M.; Hasdai, D.; Husted, S.; James, S. K.; Kervinen, K.; Kolh, P.; Kristensen, S. D.; Lancellotti, P.; Maggioni, A. P.; Piepoli, M. F.; Pries, A. R.; Romeo, F.; Ryden, L.; Simoons, M. L.; Sirnes, P. A.; Steg, P. G.; Timmis, A.; Wijns, W.; Windecker, S.; Yildirir, A.; Zamorano, J. L., 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013, 34, (38), 2949-3003.
- Douglas, P. S.; Pontone, G.; Hlatky, M. A.; Patel, M. R.; Norgaard, B. L.; Byrne, R. A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; Hink, U.; Schuchlenz, H. W.; Feuchtner, G.; Gilard, M.; Andreini, D.; Jensen, J. M.; Hadamitzky, M.; Chiswell, K.; Cyr, D.; Wilk, A.; Wang, F.; Rogers, C.; De Bruyne, B.; Investigators, P., Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J 2015, 36, (47), 3359-67.
- Neumann, F. J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A. P.; Benedetto, U.; Byrne, R. A.; Collet, J. P.; Falk, V.; Head, S. J.; Juni, P.; Kastrati, A.; Koller, A.; Kristensen, S. D.; Niebauer, J.; Richter, D. J.; Seferovic, P. M.; Sibbing, D.; Stefanini, G. G.; Windecker, S.; Yadav, R.; Zembala, M. O.; Group, E. S. C. S. D., 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019, 40, (2), 87-165.
- Norgaard, B. L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B. S.; Ito, H.; Jensen, J. M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; Osawa, K.; Marwan, M.; Naber, C.; Erglis, A.; Park, S. J.; Christiansen, E. H.; Kaltoft, A.; Lassen, J. F.; Botker, H. E.; Achenbach, S.; Group, N. X. T. T. S., Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014, 63, (12), 1145-1155.
- Cook, C. M.; Petraco, R.; Shun-Shin, M. J.; Ahmad, Y.; Nijjer, S.; Al-Lamee, R.; Kikuta, Y.; Shiono, Y.; Mayet, J.; Francis, D. P.; Sen, S.; Davies, J. E., Diagnostic Accuracy of Computed Tomography-Derived Fractional Flow Reserve : A Systematic Review. JAMA Cardiol 2017, 2, (7), 803-810.
- Finlay, D. D.; Nugent, C. D.; Donnelly, M. P.; McCullagh, P. J.; Black, N. D., Optimal electrocardiographic lead systems: practical scenarios in smart clothing and wearable health systems. IEEE Trans Inf Technol Biomed 2008, 12, (4), 433-41.
- Baek, J.-Y.; An, J.-H.; Choi, J.-M.; Park, K.-S.; Lee, S.-H., Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sensors and Actuators A: Physical 2008, 143, (2), 423-429.
- Haghdoost, F.; Mottaghitalab, V.; Haghi, A. K., Comfortable textile-based electrode for wearable electrocardiogram. Sensor Review 2015, 35, (1), 20-29.
- Chlaihawi, A. A.; Narakathu, B. B.; Emamian, S.; Bazuin, B. J.; Atashbar, M. Z., Development of printed and flexible dry ECG electrodes. Sensing and Bio-Sensing Research 2018, 20, 9-15.
- Pani, D.; Dessi, A.; Saenz-Cogollo, J. F.; Barabino, G.; Fraboni, B.; Bonfiglio, A., Fully Textile, PEDOT:PSS Based Electrodes for Wearable ECG Monitoring Systems. IEEE Trans Biomed Eng 2016, 63, (3), 540-9.
- Stauffer, F.; Thielen, M.; Sauter, C.; Chardonnens, S.; Bachmann, S.; Tybrandt, K.; Peters, C.; Hierold, C.; Voros, J., Skin Conformal Polymer Electrodes for Clinical ECG and EEG Recordings. Adv Healthc Mater 2018, 7, (7), e1700994.
- Chen, Y. H.; Op de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlovic, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C., Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors (Basel) 2014, 14, (12), 23758-80.
- Joosten, A.; Desebbe, O.; Suehiro, K.; Murphy, L. S.; Essiet, M.; Alexander, B.; Fischer, M. O.; Barvais, L.; Van Obbergh, L.; Maucort-Boulch, D.; Cannesson, M., Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: a systematic review and meta-analysisdagger. Br J Anaesth 2017, 118, (3), 298-310.
- Ma, Y.; Choi, J.; Hourlier-Fargette, A.; Xue, Y.; Chung, H. U.; Lee, J. Y.; Wang, X.; Xie, Z.; Kang, D.; Wang, H.; Han, S.; Kang, S. K.; Kang, Y.; Yu, X.; Slepian, M. J.; Raj, M. S.; Model, J. B.; Feng, X.; Ghaffari, R.; Rogers, J. A.; Huang, Y., Relation between blood pressure and pulse wave velocity for human arteries. Proc Natl Acad Sci U S A 2018, 115, (44), 11144-11149.
- Meijboom, W. B.; Van Mieghem, C. A.; van Pelt, N.; Weustink, A.; Pugliese, F.; Mollet, N. R.; Boersma, E.; Regar, E.; van Geuns, R. J.; de Jaegere, P. J.; Serruys, P. W.; Krestin, G. P.; de Feyter, P. J., Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008, 52, (8), 636-43.
- Ale, I. S.; Maibacht, H. A., Diagnostic approach in allergic and irritant contact dermatitis. Expert Rev Clin Immunol 2010, 6, (2), 291-310.
- Brasch, J.; Becker, D.; Aberer, W.; Bircher, A.; Kranke, B.; Jung, K.; Przybilla, B.; Biedermann, T.; Werfel, T.; John, S. M.; Elsner, P.; Diepgen, T.; Trautmann, A.; Merk, H. F.; Fuchs, T.; Schnuch, A., Guideline contact dermatitis: S1-Guidelines of the German Contact Allergy Group (DKG) of the German Dermatology Society (DDG), the Information Network of Dermatological Clinics (IVDK), the German Society for Allergology and Clinical Immunology (DGAKI), the Working Group for Occupational and Environmental Dermatology (ABD) of the DDG, the Medical Association of German Allergologists (AeDA), the Professional Association of German Dermatologists (BVDD) and the DDG. Allergo J Int 2014, 23, (4), 126-138.
- Jin, H.; Abu-Raya, Y. S.; Haick, H., Advanced Materials for Health Monitoring with Skin-Based Wearable Devices. Adv Healthc Mater 2017, 6, (11).
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y., Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors (Basel) 2018, 18, (2).
- Park, M.; Bok, B. G.; Ahn, J. H.; Kim, M. S., Recent Advances in Tactile Sensing Technology. Micromachines (Basel) 2018, 9, (7), 321.
- Haddara, Y. M.; Howlader, M. M. R., Integration of Heterogeneous Materials for Wearable Sensors. Polymers (Basel) 2018, 10, (1), 60.
- Lee, Y. H.; Jang, M.; Lee, M. Y.; Kweon, O. Y.; Oh, J. H., Flexible Field-Effect Transistor-Type Sensors Based on Conjugated Molecules. Chem 2017, 3, (5), 724-763.
- Pereni, C. I.; Zhao, Q.; Liu, Y.; Abel, E., Surface free energy effect on bacterial retention. Colloids Surf B Biointerfaces 2006, 48, (2), 143-7.
- Gusnaniar, N.; van der Mei, H. C.; Qu, W.; Nuryastuti, T.; Hooymans, J. M. M.; Sjollema, J.; Busscher, H. J., Physico-chemistry of bacterial transmission versus adhesion. Adv Colloid Interface Sci 2017, 250, 15-24.
- Oh, J. Y.; Rondeau-Gagne, S.; Chiu, Y. C.; Chortos, A.; Lissel, F.; Wang, G. N.; Schroeder, B. C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; Xu, J.; Zhu, C.; Gu, X.; Bae, W. G.; Kim, Y.; Jin, L.; Chung, J. W.; Tok, J. B.; Bao, Z., Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, (7629), 411-415.
- Lee, Y.; Park, J.; Choe, A.; Cho, S.; Kim, J.; Ko, H., Mimicking Human and Biological Skins for Multifunctional Skin Electronics. Advanced Functional Materials 2019, n/a, (n/a), 1904523.
- Shimizu, R.; Nonomura, Y., Preparation of Artificial Skin that Mimics Human Skin Surface and Mechanical Properties. J Oleo Sci 2018, 67, (1), 47-54.
- Wang, T.; Si, Y.; Luo, S.; Dong, Z.; Jiang, L., Wettability manipulation of overflow behavior via vesicle surfactant for water-proof surface cleaning. Materials Horizons 2019, 6, (2), 294-301.
- Stadlober, B.; Zirkl, M.; Irimia-Vladu, M., Route towards sustainable smart sensors: ferroelectric polyvinylidene fluoride-based materials and their integration in flexible electronics. Chem Soc Rev 2019, 48, (6), 1787-1825.
- Santarelli, L., Organic Semiconductors-Based Devices Electrical Reliability to Environmental Stress. 2018.
- Xu, K.; Lu, Y.; Takei, K., Multifunctional Skin-Inspired Flexible Sensor Systems for Wearable Electronics. Advanced Materials Technologies 2019, 4, (3), 1800628.
- Kenry; Yeo, J. C.; Lim, C. T., Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst Nanoeng 2016, 2, (1), 16043.
- Patrick, J. F.; Robb, M. J.; Sottos, N. R.; Moore, J. S.; White, S. R., Polymers with autonomous life-cycle control. Nature 2016, 540, (7633), 363-370.