Rechargeable alkali metal-air batteries have enormous potential in energy storage applications due to their high energy densities, low-cost and environment friendliness. Membrane separators determine the performance and economic viability of these batteries. Usually, porous membrane separators taken from lithium-based batteries are used. Moreover, composite and cation-exchange membranes have been tested. However, crossover of unwanted species (such as zincate ions in zinc-air flow batteries) and/or low hydroxide ions conductivity are major issues to be overcome. On the other hand, state-of-art Anion-Exchange Membranes (AEMs) have been applied to meet the current challenges with regard to rechargeable zinc-air batteries, which has received the most attention among alkali metal-air batteries. The recent advances and remaining challenges of AEMs for these batteries are critically discussed. Correlation between the properties of the AEMs and performance and cyclability of the batteries has been established.
- Alkali metal-air batteries
- Zn-air batteries
- Anion-exchange membranes
- Recent advances
A literature survey was done and analyzed to understand the weaknesses and strengths of the AEMs reported in the literature and commercial AEMs, such as A201® (Tokuyama Corporation, Japan) and FAA-3® (FumaTech, Germany) when used in alkali metal-air batteries. FAA®-3 is a slightly cross-linked, non-reinforced AEM consisting of a polyaromatic backbone with a quaternary ammonium group. FAA®-3-50 is 45–55 µm thick and has an ion-exchange capacity (IEC) of 2 meq/g in chloride form[1][2][3]. A201® (IEC = 1.7 mmol/g and 28 µm thick) employees quaternary ammonium groups and hydrocarbon main chain[4][5]. AEMs used in Zn–air batteries are mainly discussed, as these batteries are better explored compared to the others. Among the commercial AEMs, A201® membrane has been most tested in rechargeable Zn–air batteries. Moreover, in addition to commercial AEMs, preparation of AEMs for Zn–air batteries have been reported in the literature. The cycling stabilities of the batteries have been found to be dependent on ionic conductivity, zincate diffusion (selectivity), water uptake capacity, and anisotropic swelling ratio of the membranes. Anisotropic swelling of membranes is defined as the ratio of through-plane to in-plane swelling of the membrane. Moreover, prospects on the possible use of other commercial AEMs have also been investigated.
Recently, Abbasi et al.[6] prepared poly (p-phenylene oxide) (PPO)-based AEMs using three different cations—trimethylamine (TMA), 1-methylpyrolidine (MPY), and 1-methylimidazole (MIM)—and tested them in a Zn–air battery. PPO-TMA and PPO-MPY exhibited low zincate diffusion coefficients (1.13 × 10−8, and 0.28 × 10−8 cm2/min, respectively) and high discharge capacity (about ~800 mAh/gZn using PPO-TMA). The PPO-TMA membrane was reported to have low conductivity (0.17 mS/cm) despite its high water uptake (89 wt.%). On the other hand, the membranes showed good alkaline stability in a solution typically used in Zn–air batteries (7 M KOH solution at 30 °C) for at least 150 h. Moreover, PPO-TMA showed good electrochemical stability in a range of −1.5 to +1.5 V (stability window of 3 V). It is a well-established fact that PPO-TMA membranes undergo an SN2 hydroxide attack[7]. In this degradation process, the C–N bond electrons move towards the nitrogen while the OH− forms a new bond with the α carbon, producing trimethylamine and benzyl alcohol. Therefore, the relative alkaline stability of the current membranes could be due to the low temperature and the reasonable duration of the test.
In another study, a polysulfonium-cation-based AEM was fabricated and used in a Zn–air battery[8]. Compared to Celgard® 5550, the prepared membrane demonstrated better ionic selectivity. As a result, the capacity was 6-fold higher than that of the reference membrane during discharge. However, the species crossing over was mistakenly considered to be Zn2+, rather than Zn(OH)42−. Moreover, the cyclability of the battery was not studied.
A porous alkaline-exchange membrane based on quaternary ammonium (QA)-functionalized nanocellulose (2-QAFC, cellulose nanofibres modified with 200 mol. % concentration of dimethyloctadecyl [3-(trimethoxysilyl) propy l] ammonium chloride) exhibiting high hydroxide ion conductivity (21.2 mS/cm) and water swelling (95.6%) was developed[9]. Figure 1 presents the procedure followed for the preparation of the 2-QAFC membrane (Figure 1a), galvanostatic discharge of solid-state Zn–air batteries using the 2-QAFC, A201® and KOH-PC membranes (1 M KOH-doped pristine cellulose membranes) (Figure 1b), and galvanostatic charge and discharge cycling (Figure 1c). Both the prepared 2-QAFC (30 µm thickness) and commercial A201® membranes (28 µm thickness) were tested in a flexible, solid-state rechargeable Zn–air battery. Initially, the A201®-based battery had a higher discharge voltage; however, it was quickly surpassed by the 2-QAFC-based battery, showing a voltage plateau about 180 mV higher and higher discharge capacity.
The A201®-based battery exhibited a rapid voltage and capacity loss, which could have been due to the progressive loss of water and ionic conductivity in the membrane during the constant current applied. It seems that water consumption during oxygen reduction in air electrode leads to electrolyte drying problems and a shortened battery life. Since water plays an important role in ion transport, its loss can directly reduce ionic transport limitation inside the air electrode (decrease of the OH− mobility, degrading the catalyst/electrolyte interface) and inside the membrane, resulting in a large ohmic polarization of the battery. Nevertheless, it must be noted that by wetting the membrane with distilled, de-ionized water, it is possible to regenerate the performance of the battery. As a consequence, the A201®-based battery deteriorated after a few cycles (Figure 1c), showing large discharge and charge polarization.
On the other hand, the 2-QAFC-based battery exhibited superior cycling stability in both the charge and discharge (Figure 1c). This superior cycling stability was assumed to be due to the battery’s holding a higher amount of water (95.6%, OH- form) and having a smaller anisotropic swelling ratio (1.1) of 2-QAFC than A201® membrane (44.3% water uptake and 4.4 anisotropic swelling ratio). In other words, the 2-QAFC membrane could tolerate the periodic stress and dehydration during the discharge and charge processes.
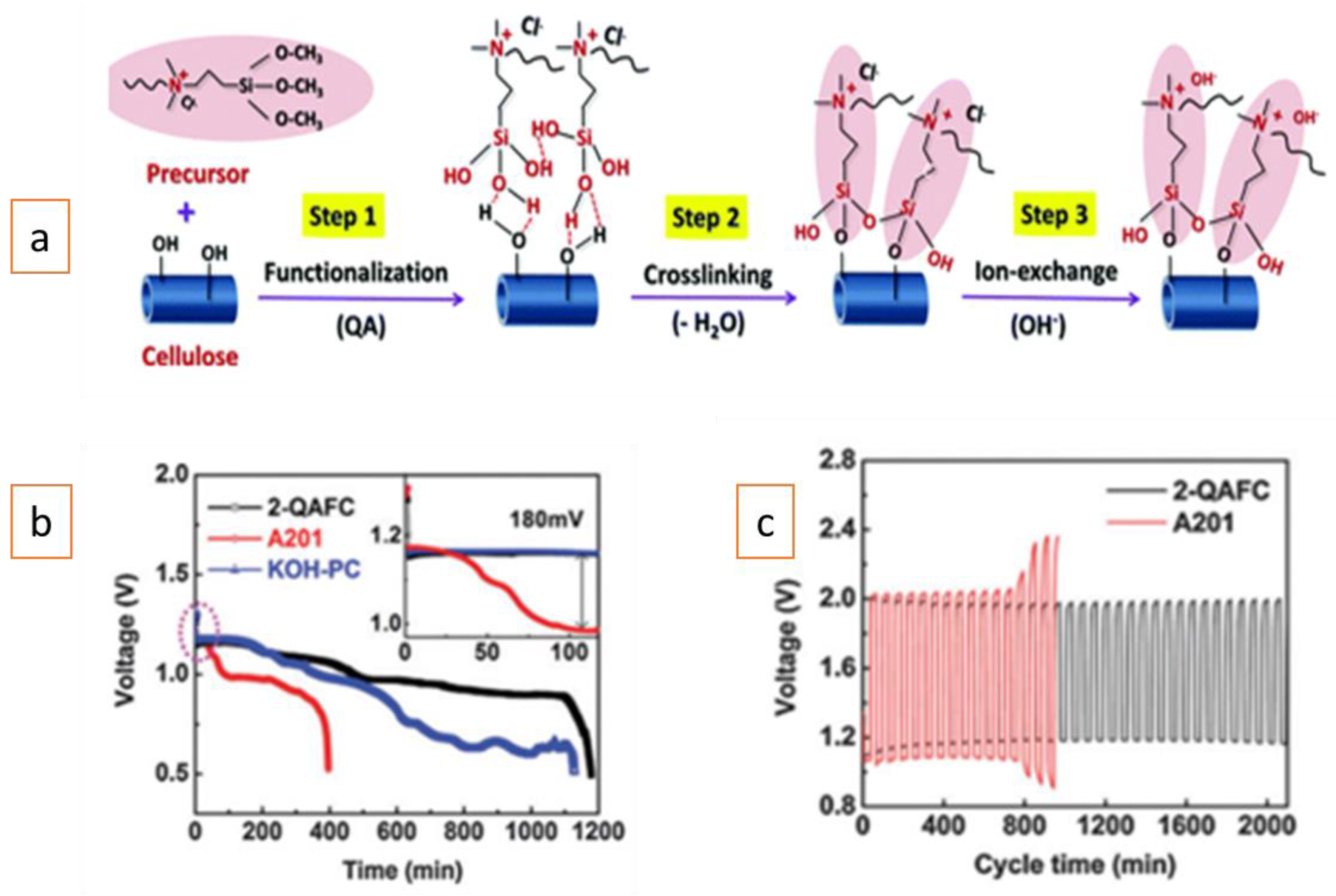
Figure 1. (a) Schematic diagram of the chemical structure evolution of the nanocellulose membrane by functionalization, cross-linking, and hydroxide exchange. (b) Galvanostatic discharge of solid-state Zn–air batteries using the 2-QAFC, A201®, and KOH-PC membranes at a current density of 25 mA/g. (c) Galvanostatic charge and discharge cycling of the 2-QAFC and A201® membranes at a current density of 250 mA/g with a 60 min per cycle period. Reprinted with permission from Reference[9]. Copyright 2016, Royal Society of Chemistry.
Similarly, a QA-functionalized, crosslinked nanocellulose/graphene oxide (QAFCGO) membrane was prepared and assembled in a flexible rechargeable Zn–air battery [10]. Batteries employing the QAFCGO and A201® membranes exhibited similar high open-circuit voltages (≈1.4 V). The QAFCGO-based battery showed a better performance compared to the A201®-based battery, with smaller overpotentials for both discharge and charge processes. At high current densities (above 20 mA/cm2), the QAFCGO-based battery showed a remarkable advantage over the A201®-based battery (Figure 2a). As shown in Figure 2b, the QAFCGO-based battery exhibited much higher cycling stability performance than that of A201®-based battery. Furthermore, the former battery had higher peak power density (44.1 mW/cm2) than the latter (33.2 mW/cm2) (Figure 2c).
Similarly to the finding of Fu et al.[9], the A201®-based battery showed a clear performance decline (with large charge and discharge polarizations) after relatively few cycles (Figure 2b). On the other hand, the QAFCGO-based battery was reported to continue without any sign of performance loss after 30 cycles. As clearly noted previously, the superior cyclability and performance stability of the QAFCGO-based battery compared to the A201®-based battery was attributed to the QAFCGO membrane’s higher water uptake (5 times higher than that of the A201® membrane) and smaller anisotropic swelling degree (half of that of the A201® membrane).
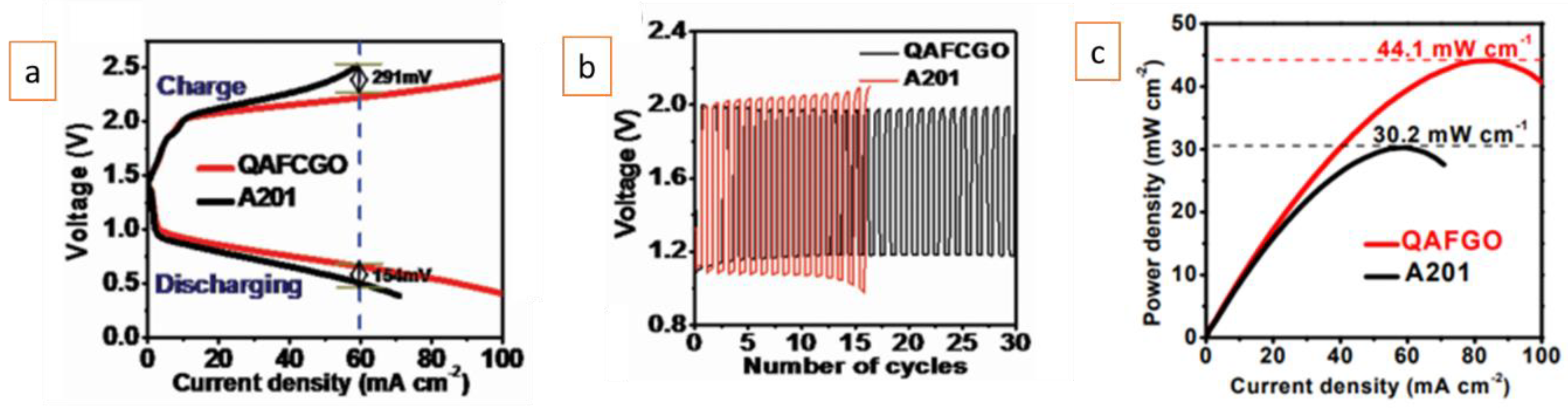
Figure 2. QAFCGO- and A201®-membrane-based Zn–air batteries: (a) charge and discharge polarization curves, (b) galvanostatic charge and discharge cycling at a current density of 1 mA/cm2 with a 20 min per cycle period (10 min discharge followed by 10 min charge), and (c) the power density plots at a current density of 1 mA/cm2. Reproduced with permission from Reference[10]. Copyright 2016, Wiley-VCH.
Furthermore, AEMs composed of both cross-linked chitosan (CS) and poly (diallyldimethylammonium chloride) (PDDA) and A201® membranes were tested in all-solid-state Zn–air batteries[11] (Figure 3a). The prepared CS-PDDA membrane exhibited high OH− conductivity (24 mS/cm), strong alkaline stability (216 h at 8 M KOH), and a low degree of anisotropic swelling (1.7), all of which are very important membrane properties required for long-term and superior electrochemical performance in all-solid-state Zn–air battery. The CS-PDDA-based battery exhibited a high open-circuit voltage (1.3 V) and superior peak power density to the A201®-based cell (48.9 vs 41.4 mW/cm2) under the same measurement conditions (Figure 3b). Additionally, the CS-PDDA-based battery initially had a higher discharge voltage (1.14 vs. 1 V), and exhibited lower discharge and charge polarization and longer cycle times (even if only a few cycles were shown) than the battery with the commercial A201® membrane (Figure 3c,d).
As mentioned in previous studies[9][10], the superior performance of the prepared membrane over A201® was due to its smaller anisotropic swelling and higher water uptake (4 times higher water uptake than the A201® membrane). It should be noted that the anisotropic swelling ratio of commercial A201® membrane was different in all the studies, indicating the lack of a standardized testing protocol.
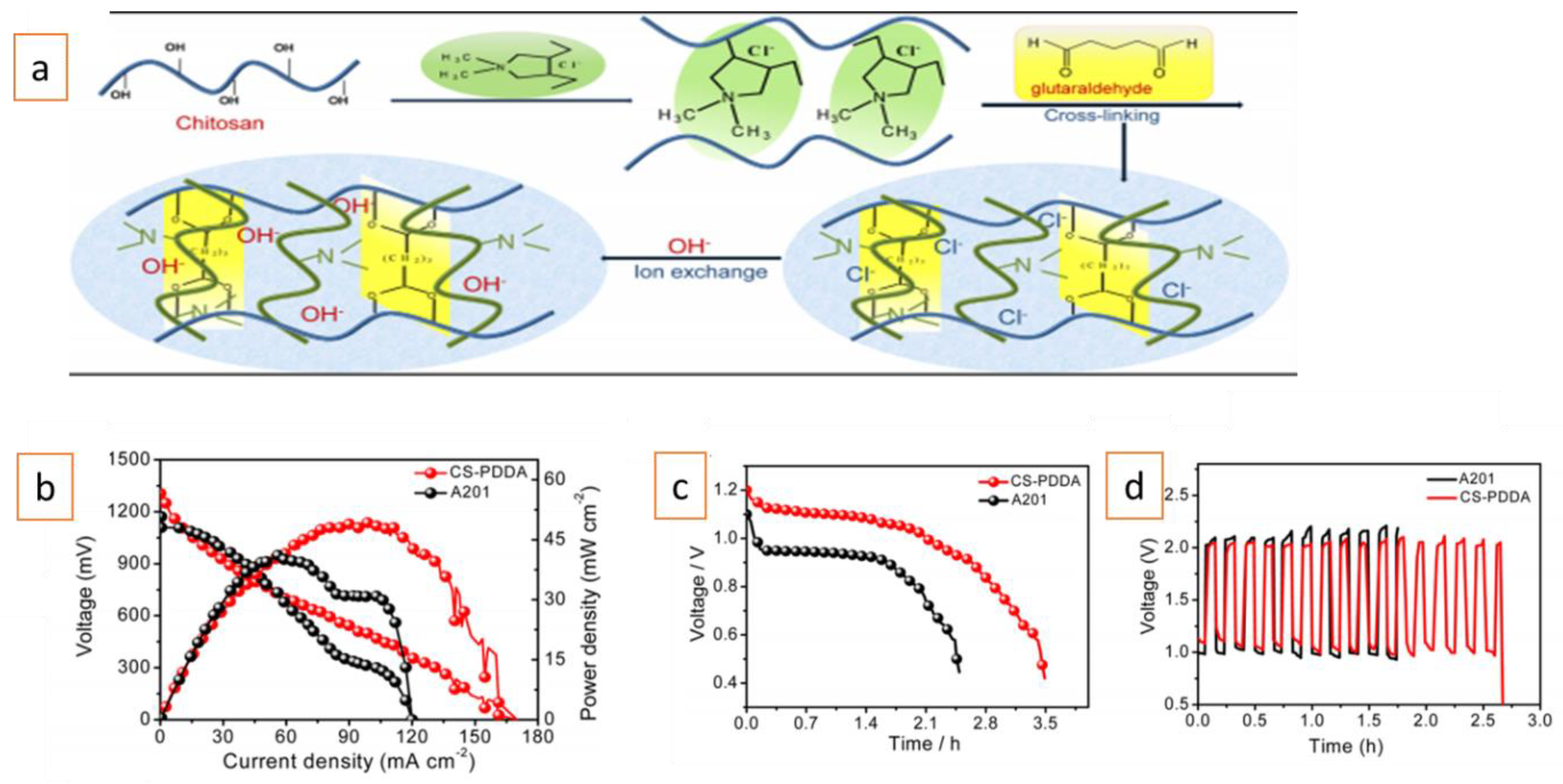
Figure 3. (a) Schematic diagram of the overall preparation procedure for the CS-PDDA-OH– membrane; (b) polarization curve and corresponding power density plots; (c) galvanostatic discharge of the batteries; (d) galvanostatic charge and discharge cycling of the batteries using the CS-PDDA-OH− and A201® membranes at 3 mA/cm2 with 10 min per cycle period. Adapted with permission from Reference[11]. Copyright 2018, American Chemical Society.
Moreover, in addition to polysulfonium and QAs, imidazolium cations have been used to prepare AEMs for Zn–air batteries. Zarrin et al.[12] prepared a graphene oxide membrane functionalized with 1-hexyl-3-methylimidazolium chloride molecules (HMIM/GO) with potential for wearable electronics, including flexible Zn–air batteries. The prepared 5-HMIM/GO (5 refers to weight ratio of HMIM to GO, 27 μm) and A201® membranes were tested in flexible Zn–air batteries. The 5-HMIM/GO membrane was reported to have a hydroxide conductivity of 44 mS/cm at room temperature and 30% relative humidity. Both membranes exhibited stable charge/discharge performances for 60 cycles. The 5-HMIM/GO-membrane-based flexible Zn–air battery exhibited a charge-discharge voltage polarization at low relative humidity and room temperature that was comparable to that of A201®-based battery in a humidified environment. This was attributed to the high rate of ion transfer of the former membrane in the studied conditions.
All in all, all the presented studies indicate the need for the development of an alkaline AEM with high hydroxide conductivity (at room temperature) and smaller anisotropic swelling degree. It can be concluded that the A201® membrane may not be practical for long-term rechargeable Zn–air batteries; in the studied systems, it was reported that its performance began to deteriorate after only a few hours. Therefore, in addition to preparing new, high-performing AEMs, testing other commercially available AEMs should be done to understand and determine their potential applications in such batteries.
Alkaline AEMs from Fumatech BWT GmbH (typically, fumapem® FAA and fumasep® FAP) are suggested by the company to be suitable separators for Zn–air batteries[13]. However, there have not been many studies in the literature to date about their practical use. Anion-exchange polymer (AEP) resin (FAA®-3-SOLUT-10 in NMP, Fumatech BWT GmbH) was used to prepare a separator and used as the separator to prepare transparent, bendable secondary Zn–air batteries[14]. The membrane was prepared using a solution (10% of AEP solution) casting method. The produced battery exhibited a maximum power density of 9.77 mW/cm2. The cells were reported to be stable for at least 100 cycles. In another study, a fumatech®-FAA membrane doped with KOH was used to prepare the membrane electrode assembly for a Zn–air battery[15]. The battery exhibited a peak power density of 170 and 164 mW/cm2 based on Fe-LC-900 (FeCl3–leather, pyrolysis temperatures of 900 °C) and Pt/C-catalyst-based air electrodes, respectively. However, in both studies, not much information was provided regarding the effects, weakness, and strength of the membranes used.
According to the technical datasheet provided by the company, fumapem® FAA-3-50 membrane, in its OH− form, has 40 wt. % H2O uptake and a dimensional swelling (in H2O) of 17% at 25 °C[16]. The membrane’s in-plane swelling ratio, and thus anisotropic swelling ratio, has not been reported to date. All in all, considering its relatively low water uptake, low performance can be expected. This is due to potential periodic stress and dehydration of the membrane, similarly to A201® membrane. However, testing in a real system is the only way to observe and understand its real strengths and weaknesses.
This publication can be found here: https://www.mdpi.com/1996-1073/12/24/4702
References
- Fumatech GmbH. Available online: https://www.fumatech.com/Startseite/index.html (accessed on 5 October 2019).
- Marcelo Carmo; Gustavo Doubek; Ryan C. Sekol; Marcelo Linardi; André D. Taylor; Development and electrochemical studies of membrane electrode assemblies for polymer electrolyte alkaline fuel cells using FAA membrane and ionomer. Journal of Power Sources 2013, 230, 169-175, 10.1016/j.jpowsour.2012.12.015.
- Dekel, D.; Schuster, M.; Ash, U.; Jaouen, F. Critical Raw Materials Elimination by a Top-Down Approach to Hydrogen and Electricity Generation. 2017, pp. 1–13. Available online: https://cordis.europa.eu/project/rcn/206995/factsheet/en (accessed on 9 December 2019).
- Hiroyuki Yanagi; Kenji Fukuta; Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). 214th ECS Meeting 2008, 16, 257-262, 10.1149/1.2981860.
- Graciela C. Abuin; Esteban A. Franceschini; Patrick Nonjola; Mkhulu K. Mathe; Mmalewane Modibedi; Horacio R. Corti; A high selectivity quaternized polysulfone membrane for alkaline direct methanol fuel cells. Journal of Power Sources 2015, 279, 450-459, 10.1016/j.jpowsour.2014.12.136.
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-based hydroxide exchange separator membranes for zinc–air battery. Int. J. Mol. Sci. 2019, 20, 3678.
- Sapir Willdorf-Cohen; Abhishek N. Mondal; Dario R. Dekel; Charles E. Diesendruck; Chemical stability of poly(phenylene oxide)-based ionomers in an anion exchange-membrane fuel cell environment. Journal of Materials Chemistry A 2018, 6, 22234-22239, 10.1039/c8ta05785k.
- Eniya Listiani Dewi; Kenichi Oyaizu; Hiroyuki Nishide; Eishun Tsuchida; Cationic polysulfonium membrane as separator in zinc–air cell. Journal of Power Sources 2003, 115, 149-152, 10.1016/s0378-7753(02)00650-x.
- Jing Fu; Jing Zhang; Xueping Song; Hadis Zarrin; Xiaofei Tian; Jinli Qiao; Lathanken Rasen; Kecheng Li; Zhongwei Chen; A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc–air batteries. Energy & Environmental Science 2016, 9, 663-670, 10.1039/c5ee03404c.
- Jing Zhang; Jing Fu; Xueping Song; Gaopeng Jiang; Hadis Zarrin; Pan Xu; Kecheng Li; Aiping Yu; Zhongwei Chen; Laminated Cross-Linked Nanocellulose/Graphene Oxide Electrolyte for Flexible Rechargeable Zinc-Air Batteries. Advanced Energy Materials 2016, 6, 1600476, 10.1002/aenm.201600476.
- Yanan Wei; Min Wang; Nengneng Xu; Luwei Peng; Jianfeng Mao; Qiaojuan Gong; Jinli Qiao; Alkaline Exchange Polymer Membrane Electrolyte for High Performance of All-Solid-State Electrochemical Devices. ACS Applied Materials & Interfaces 2018, 10, 29593-29598, 10.1021/acsami.8b09545.
- Hadis Zarrin; Serubbabel Sy; Jing Fu; Gaopeng Jiang; Keunwoo Kang; Yun-Seok Jun; Aiping Yu; Michael Fowler; Zhongwei Chen; Molecular Functionalization of Graphene Oxide for Next-Generation Wearable Electronics. ACS Applied Materials & Interfaces 2016, 8, 25428-25437, 10.1021/acsami.6b06769.
- Fumasep® Membrane Types Redox-Flow-Batteries. Available online: https://www.fumatech.com (accessed on 5 October 2019).
- Ohchan Kwon; Ho Jung Hwang; Yunseong Ji; Ok Sung Jeon; Jeong Pil Kim; Chanmin Lee; Yong Gun Shul; Transparent Bendable Secondary Zinc-Air Batteries by Controlled Void Ionic Separators.. Scientific Reports 2019, 9, 3175, 10.1038/s41598-019-38552-4.
- Roby Soni; Siddheshwar N. Bhange; Sreekumar Kurungot; A 3-D nanoribbon-like Pt-free oxygen reduction reaction electrocatalyst derived from waste leather for anion exchange membrane fuel cells and zinc-air batteries.. Nanoscale 2019, 11, 7893-7902, 10.1039/c9nr00977a.
- Technical Datasheet-Fumapem® FAA-3-50. Available online: https://fuelcellstore.com/spec-sheets/fumapem-faa-3-50-technical-specifications.pdf (accessed on 8 October 2019).
