1. Biomass Valorization: A Great Opportunity
Catalysis is the branch of chemistry that has the largest influence in global economy; in fact, it generates approximately 35% of world GDP
[1]. Heterogeneous catalysis, in particular, is responsible of almost 90% of the total volume of chemical production each year
[2], making it one of the most profitable industrial sectors worldwide. Such an extended economic compartment greatly affects not only the wealth level of several nations but along the decades has largely contributed to the generation of severe environmental issues, measurable not only in terms of greenhouse emissions (chemical related industry is the second largest industrial producer of greenhouse gases, after the metallurgical compartment)
[3] but also in accidental/volunteer release of toxic substances into the environment. Containment actions have already been taken, since it will take years to limit the pollution level generated worldwide, such that energy production is gradually abandoning fossil sources at the advantage of more sustainable ones. Alongside the most commonly known solar, wind and hydroelectric powers, energy produced by exploiting biomasses is becoming one of the key players in the field of renewable feedstocks, since they exploit natural resources more efficiently, generating a series of useful and profitable by-products and are among the cleanest sources of energy as their production and processing generates very little polluting residues
[4].
Nowadays, bioenergy covers almost 50% of all the renewable sources of energy
[5]; in the EU, its employment have risen 1.94 times in the past 15 years
[6] and this trend is expected to continue in the next few decades. At the industrial level, companies are more likely to employ raw materials as homogeneously as possible, in order to maximize process yields and consequently incomes: therefore, natural oils (such as canola, soy, sunflower or palm oil) are harvested with the sole purpose of being transformed into fatty acid methyl esters (FAME hereafter) to be employed as fuels, contributing significantly to the overall goal of adopting renewable and zero-emission energy sources
[7]. However, despite this practice preventing the release of greenhouse gases from fossil feedstocks into the atmosphere, it is increasing land-grabbing phenomena in developing countries where these plants are effectively grown
[8], tremendously endangering the local population and wildlife. The problems risen by using cultivated feedstocks as renewable sources can be somehow limited by the partial substitution of these raw materials with organic wastes from human activities, thus transforming substances that need to be disposed into precious feedstocks.
With this review paper, we are trying to build a bridge between the highly interesting topic of catalytic biomass valorization and the study of the influence that supports may exert on the total activity and selectivity of heterogeneous catalysts. In the introductory section, we will gather as high an amount of available information about biomasses discarded by human activities as possible, rationalizing and dividing them according to their origin, chemical composition and trying to provide a wide overview of their potentiality of employment in the industrial chemical sector. However, we are well aware of the huge size of this topic; therefore, we will limit our focus to the applications in the catalytic field. Quite often in modern green chemistry, biomasses are employed as catalysts or as supports, either directly (chitosan) or after a chemical transformation (activated carbons and lignin), thanks to their natural abundance of chemical species able to either directly perform directly catalyzed reactions or able to coordinate metal ions and nanoparticles. In the latter case, such functionalities might influence the reaction path, acting as directing agents towards certain products; also, the use of pure and transformed biomasses as supports will be explored in the introductory part of the paper. In
Section 3, we will report those research papers that have investigated the possible influence that the structural and surface characteristics of materials have on the total catalytic activity when employed for supporting heterogeneous catalyst. As for
Section 2, our focus will be on reactions involving biomass valorization, from more classical oxide-supported catalysts to MOFs, 2D materials and nanoparticles supported active phases.
2. Biomass from Wastes to Feedstocks: Typologies, Composition, Advantages and Drawbacks
Biomasses of different origins, strictly intended as polysaccharidic material obtained by vegetal organisms via photosynthetic route
[9], have been employed since the rise of the first agricultural societies for purposes that are diverse from their primary intended use (namely as food source for human beings or cattle): wood from forests have been used for building shelters or as fuel; the same for wastes deriving from crops, but they have also been employed as fertilizers, together with animal manure. With the development of industrial society in the 18th century, united with modern building technologies, progresses in the field of chemistry and the progressive mechanization of agricultural processes, the importance of such natural feedstocks for technological applications started to decline, touching its minimum during fossil fuels era along the 20th century. Luckily, starting from the 1970s a growing awareness among people and national governments about the environmental disaster that planet Earth was facing due to the indiscriminate use of non-renewable sources was born
[10]. This pushed the society to find a way to bring back to play an enormous amount of unexploited sources that we were simply throwing away. However, the idea of using again biomasses in the same way they were employed centuries ago was definitely outdated, also for the different needs and technologies modern society has with respect to the past; moreover, other biomasses obtained by waste materials of new food industry processes (and not available only few decades ago) entered the field. According to Tursi
[11] and Kaltschmitt
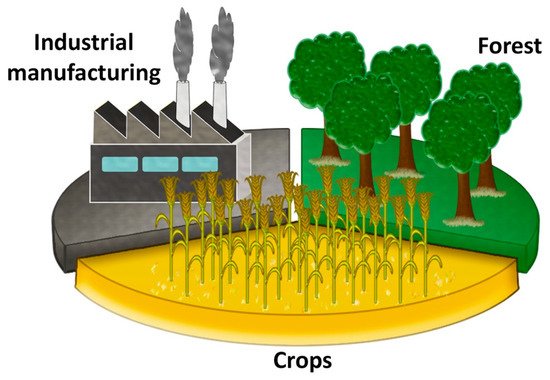
[12], there are three main areas where biomasses from waste can be “harvested” (see
Figure 1): the most important are the ones of agricultural activities and forestry residues, including residues coming from their industrial manufacturing (such as peels, shells, wood shavings and sawdust). In terms of quantity, they are closely followed by animal residues from livestock farms, sewage, algae and aquatic crops. Urban solid wastes and in general wastes originating from anthropogenic activities also fall in the biomass category, but only if they are not reusable in subsequent processing.
Figure 1. Principal vegetal biomass sources according to Tursi
[11] and Kaltschmitt
[12].
As previously mentioned, the main problem with biomass wastes stands behind their heterogeneity, not only among different classes, but also within the same one; therefore, depending on the desired application, some categories are preferred with respect to others. For simple uses, such as domestic fuel, any type of biomass (properly dried in order to remove excess water) can be employed almost without any processing, thanks to their intrinsic high carbon content. This final use, which is the most primitive, varies significantly according to the wealth level of the nations: in third world nations, this type of energy accounts up to 50% of the total, against an average of 11% worldwide
[10]. Nevertheless, also among the nations with the highest GDP per capita these data are not homogeneous, with the United States delivering only 3% of the country’s energy demands by using biomass, while other countries such as Finland, Sweden, and Austria are quite above the EU average (3.5%), producing 18%, 17%, and 13% of their total energy from biomass sources
[13]. Despite the easiness of employment, the energetic application (together with the fertilizing use) results to be the sector with the lowest added value obtainable from these complex feedstocks. From an historical point of view, the first valuable product that was obtained by plant biomasses was paper. In the beginning of the pharmaceutical and cosmetic industrial story, many of their processes were based on molecules that can be found in various concentrations inside numerous natural matrices, mainly plant based. However, together with the progresses of the chemical industry, the tendency has been to move more and more towards molecules of synthetic origin, obtained by non-renewable more homogeneous feedstocks. Along the years, with the exponential rise of the costs of fossil materials from the 1970s
[14], together with one of the wastes disposal, the concept of substituting oil-based sources with biomass wastes has become economically interesting and sustainable, even if this is not valid for any possible chemical.
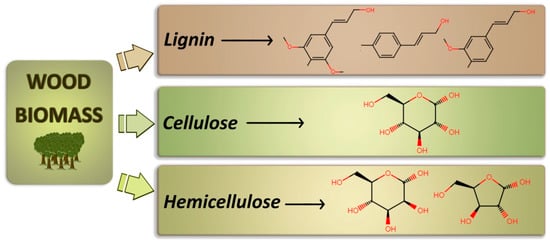
Concerning the compositional point of view, all plant-based biomasses are formed by three major constituents, namely cellulose, hemicellulose and lignin, present in different ratios depending on biomass origin (see
Figure 2)
[15]. Among the three, cellulose is the main component, and it is formed by extremely long linear chains of polysaccharides (D-Pyranglucose units linked by β-1,4-Glycosidic bonds
[16]) arranged either in crystalline or amorphous structures, which ratio (usually between 30 and 80%) depends on the average chain length
[17]. By far, cellulose is also the source exploited for the longest period of time, being the main constituent in the production of paper that is still its main application. After cellulose, hemicellulose represents the second major constituent of plant cell walls and consists of heterogeneous branched chains of polysaccharides. The amount of hemicellulose and its structure are strongly dependent on the type of vegetal source
[18]. The different saccharide units are arranged with various substituents and a diverse ratio. From hemicellulose decomposition non-condensable gas, coal, and a variety of ketones, aldehydes, acids, and furans are produced
[19]. Differently from cellulose, the hemicellulose chain structure consists of a mixture of sugars containing either five carbon atoms, such as xylose and arabinose, or six carbon atoms, such as glucose, galactose, mannose and rhamnose, with an average maximum molecular weight of 30,000 Da, definitely much lower with respect to the several millions of Da in cellulose chains
[20]. From these constituents, it is possible to obtain several different types of molecules, including xylans, mannans, galactans, and arabinogalactans. The last constituent of vegetal biomass is lignin, contained in plant cell walls similarly to the previous ones. Its role is linking the fibers of cellulose and hemicellulose together, enhancing the resistance of the plant structure. Due to its binder role, it has to be either removed or degraded in order to extract cellulosic fibers from vegetal biomasses. Its content in the materials varies according to the type of plant and its age (from 15 to 50%); however, the elemental composition is approximately always identical, consisting of 61–65% carbon, 5–6% hydrogen and 29–34% oxygen
[21]. The structure of lignin is quite far from regularity: indeed, it is a complex amorphous aromatic polymer with a three-dimensional network, with monomeric units (principally aromatic alcohols with different degrees of methoxylation
[22]) bound together by oxygen bridges. Furthermore, in the lignin structure, the presence of a high number of polar groups and hydroxyl groups allows the formation of strong intermolecular and intramolecular hydrogen bonds, making it insoluble in most solvents. Being so difficultly workable and having a greater calorific value compared to lignocellulose biomass
[23], only about 2% of the lignin produced in the world as waste material (300 billion tons
[24]) is employed in the production of various value-added products, while the rest is employed as fuel. Therefore, there is a lot of margin for the development of innovative technologies for its valorization.
Figure 2. Graphical representation of the main chemical components of vegetal biomasses, i.e., cellulose, hemicellulose and lignin, with schematized molecular sub-units.
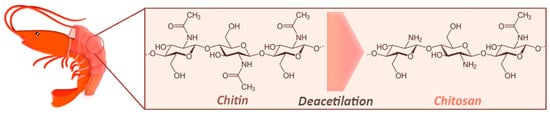
Up to this point, we discussed prevalent biomasses coming from the vegetal world. However, the alimentary industry involves also the production of animal-based food, leaving a series of byproducts that may be exploited in the chemical industry to produce higher value products. The transformation of seafood (especially shellfish) ends up with enormous quantity of organic waste, almost entirely composed by chitin
[25][26]: it is a hydrophobic polysaccharide made from acetyl-glucosamine and glucosamine grafts found in the outer skeleton of shrimps, squids, lobsters, crabs or walls of algae
[27][28][29], showing many properties including good biodegradability and biocompatibility
[30][31]. Chitin itself does not have enormous impact on the chemical industry, which significantly prefers to employ chitosan (see
Figure 3), obtained through the deacetylation of chitin
[32][33][34], which has attracted a lot attention in the green nanotechnology field. The benefits of using chitosan in technological applications include: (i) its low-cost and eco-friendly nature, (ii) its hydrophilic character, (iii) its proneness to chemical and physical modifications to customize its properties (with the insertion of coordinating groups for metal ions), and (iv) its thermal stability up to 280 °C, nontoxicity and biodegradability
[28][35][36][37][38][39][40].
Figure 3. Chitosan production chain, from chitin extraction from shellfish exoskeleton to its deacetylation process to obtain chitosan.
3. Classic Supports vs. Modern Active Supports
Once we have defined the source of feedstocks for our chemicals, it is important to go deeper into their utilization. In order to convert these materials into valuable products, the use of catalysts is often needed. It is well established that homogeneous ones can be designed to possess high selectivity towards a selected product, but the disadvantage of the non-reusability is pushing in the direction of heterogeneous counterparts, if valid alternatives are available. It is hard to find heterogeneous catalysts constituted of self-standing materials; in fact, it is preferrable to employ supported catalysts. At the dawn of the modern chemical industry, the sole role of the support was to disperse active phase, preventing its aggregation, and avoiding interferences in the reaction. Silica, alumina and carbons have been for decades as the key players in the heterogeneous supports’ world. In modern times, the shortage of feedstocks and environmental issues are forcing the production of more efficient catalysts. One way is through research focused on improving active phases. However, in some cases it is difficult (if not impossible) to improve the performances of catalysts, especially in those cases regarding noble metals; in other cases, the improvements are not environmentally or economically sustainable. For these reasons, along the years the focus has gradually moved in the direction of support development, which is usually cheaper and greener than an active species one, making the field quite attractive and with a lot of potentiality. The simplest way to modify supports properties is to act on their size and shape; oxides have been downscaled into nanoparticles, showing a great influence on the final catalytic properties, allowing a fine-tuning for preferential exposure of specific reactive sites. This can maximize the number of active sites available for reagents, improve activity, and also direct the reaction to a preferential product to achieve greater selectivity
[41]. Shape could determine which specific reactive component on the oxide particle will be exposed and this, in turn, will affect the adsorption and activation of the reactants and intermediates and, therefore, on the overall catalytic properties. The synthetic approaches available so far have allowed precise control of the size of the oxide particles with significantly improved surface densities of the active sites. However, shape control, especially for particles smaller than 10 nm is still empirical. Furthermore, the presence of surface defects located at the edges and, in some cases, also at the corners of the oxide particles, resulting from shape imperfections, can contribute to the catalytic performance. As a final consideration, the majority of classic support materials for heterogeneous catalysts were developed and optimized, in the past, for hydrocarbons-based chemicals conversion; in this context, it should be noted that oxygenated compounds, such as the ones obtained by biomass-wastes, tend to be much more aggressive towards many oxidic or carbonaceous supports, as well as towards the active phases. Harsh conditions implying that the use of water, organic polar solvents, high temperatures, strong oxidants, and prolonged reaction times, may potentially degrade classical heterogeneous supports. Whenever size and shape modification are not enough, surface functionalization can be a way to imprint peculiar properties to the catalysts, either by providing the active phase a better coordination, granting its anchoring on support surface
[42], or by favoring substrate adsorption, migration to the active species and products desorption
[43]. In those cases, in which the desired properties are not achieved with all the possible modifications of the support, the use of materials different in nature might be necessary. Providing that not all the metals are catalytically active, there is a plethora of available, or soon to be available, supports that possibly overcome the limited number of active metals or promoters that can be deposited on the supports. For this reason, an in-depth understanding of the actual role of the support in promoting the catalytic action of active phases is of paramount importance. As already described above, biomass itself provides inhomogeneous resources, thus the adaptability of the catalysts, given also by the modulation of the supports, is already a key parameter. The currently available alternatives are numerous and can span from material having natural origins to fully synthetic ones.