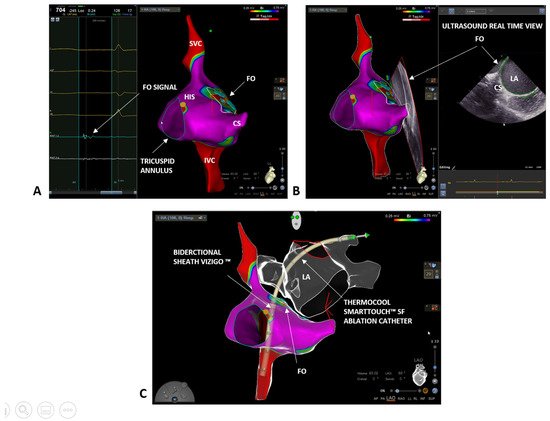
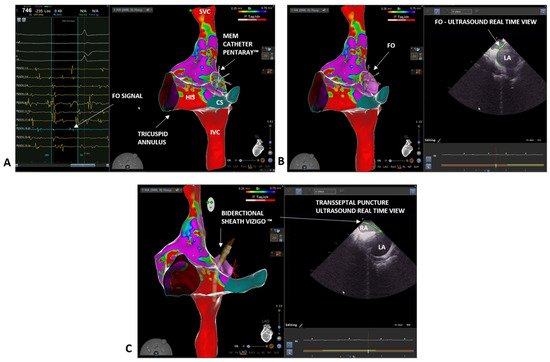
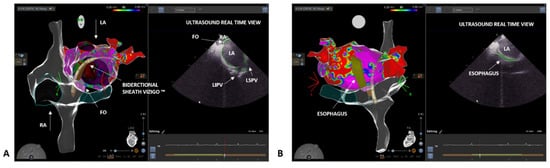
Over the last three decades, interventional cardiology has made great progress in the field of electrophysiology, particularly concerning the development of advanced techniques in electrophysiological studies and ablation procedures. Fluoroscopy-based approaches are widely validated and have been recognized as the gold standard for a long time. The use of fluoroscopy exposes both the healthcare staff and the patient to a non-negligible dose of radiation. For example, a procedure of atrial fibrillation (AF) ablation exposes patients to a dose up to 60 mSv and raises the absolute lifetime risk of a fatal cancer in an adult by 0.08% [
1]. Furthermore, the latest evidence shows that the median per year radiation exposure for interventional cardiologists is up to three times higher compared with a typical radiologist [
2]. To minimize the risks, it would be reasonable to take steps to comply with the ALARA (as low as reasonably achievable) principles, as established by an expert consensus document drawn up by the American College of Cardiology in 1998 [
3]. Advanced 3D electro-anatomic mapping (EAM) systems have transformed catheter-based ablation, allowing the electrophysiologist a new insight into the study of complex arrhythmia, such as AF and ventricular tachycardia. All mapping systems, even if based on different principles, allow a precise localization of ablation catheter from the vascular access to the heart chambers. While scanning different cardiac structures with the catheter, the system records data about catheter localization and signals information. Spatial and electrical data are used together by the system to create an accurate reproduction of the 3D geometry of cardiac structures. Remarkably, using 3D EAM systems does not necessarily mean less radiation for patients and medical staff. A multicenter prospective study showed that reduction of fluoroscopy time is associated with patient’s age, type of arrhythmia, and operator’s expertise [
4]. Despite the meaningful improvements in the field of arrhythmia mapping and ablations, ventricular tachycardia and AF still represent a great challenge for electrophysiologists. Although these arrhythmias are characterized by a greater complexity in terms of procedural maneuvers and activation mapping, newer mapping systems proved to be safe and effective approaches even for these arrhythmias [
5]. Recently, catheter ablation has become increasingly adopted for symptomatic AF. According to the European society of cardiology guidelines, pulmonary vein isolation (PVI) should/may be considered as a first-line rhythm control therapy to improve symptoms in selected patients with symptomatic paroxysmal AF episodes (IIa), or persistent AF without major risk factors for AF recurrence as an alternative to antiarrhythmic drug class I or III, considering patient choice, benefit, and risk (IIb) [
6]. Considering the worldwide spreading of this technique and the high exposure of radiation related to transseptal puncture and pulmonary vein isolation, promoting zero-fluoroscopy strategies in AF ablation is crucial to be in line with ALARA principles [
4,
7,
8]. Nowadays, the use of fluoroscopy in radiofrequency ablation of AF is markedly reduced as compared to few years ago [
9]. AF ablation procedures are often performed with low doses of fluoroscopy, but procedures with complete zero fluoroscopy are anecdotic. A workflow to reduce radiation use was recently published with satisfactory results, but this approach includes the use of transesophageal echocardiography guidance for the transseptal puncture with the need of cardiologists experienced in cardiac imaging during the ablation procedure [
10]. A complete zero-fluoroscopy approach performed by electrophysiologists is possible using intracardiac echocardiography (ICE), but it is only limited and no standardized data are present [
11]. Therefore, most of the procedures are still performed with low fluoroscopy use, mainly because transseptal maneuver is still performed under fluoroscopic guidance [
12]. A complete zero-fluoroscopy approach using the combination of ICE and visualization of transseptal sheath through 3D EAM has not been systematically described. We report our initial experience in radiofrequency AF ablation without the use of fluoroscopy (complete zero fluoroscopy), describing our workflow for both paroxysmal and persistent AF procedures. To achieve zero fluoroscopy, we use the Carto 3
® EAM system (Biosense Webster, Johnson & Johnson Medical S.p.a., Irvine, CA, USA), ICE with CartoSound
® module (Biosense Webster, Johnson & Johnson Medical S.p.a., Irvine, CA, USA), and the VIZIGO™ steerable sheath (Biosense Webster, Johnson & Johnson Medical S.p.a., Irvine, CA, USA), which can be visualized on the mapping system.