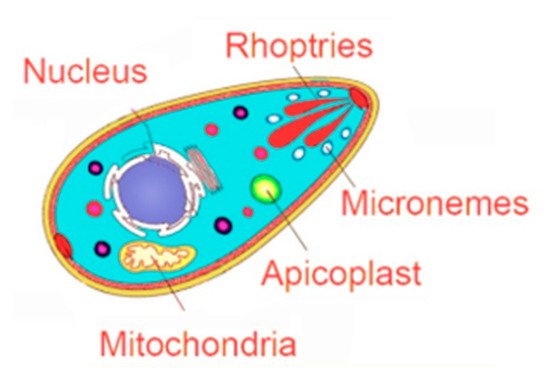
ROP2 family contains a group of proteins, with some members sharing more than 70% identity, while other members are structurally more divergent [
116]. While the members of this family evolved with all the elements to be active kinases, some members (ROP2, ROP4, ROP7, ROP5) lost some key motifs or residues in the kinase activity domain over time to acquire other functions [
116,
117]. For instance, ROP2 contributes [
118], but is not the only factor [
119], to the recruitment of the host mitochondria around the PVM. ROP5 exhibits an inverted topology in the PVM as compared to other members of the family [
120], and protein forms a complex with ROP17 and ROP18 (which retained their kinase activity), hence controlling the virulence in mice [
121,
122]. In that sense, ROP5 and ROP18 allele combinations are tightly related to
T. gondii virulence [
122,
123,
124,
125], and ROP5 teams up with ROP18 and complements its activity to inhibit the accumulation of the IFN-γ-induced immunity-related GTPases (IRGs) in vivo, hence contributing to the pathogenesis and immune evasion [
126]. Owing to the role of ROP5 and ROP18 in virulence, attempts to use this complex as a vaccine strategy were promising in mice [
127]. In addition, recombinant ROP5 and ROP18 were evaluated for their diagnostic potential in human toxoplasmosis [
128]. ROP16 and ROP18 were also proven as virulence factors through targeting the host cell nucleus and exhibiting their kinase activity to phosphorylate key proteins involved in cell cycle and different signaling pathways [
129]. ROP18 is expressed in genotypes I/II demonstrating their role in controlling the virulence of the parasite [
130], and transfection of the virulent ROP18 allele into a nonpathogenic type III strain confers virulence and enhances mortality in vivo [
131]. Through its kinase activity, ROP18 phosphorylates GTPases, promoting macrophage survival and virulence [
132] and ensuring an immune evasion strategy for virulent strains [
133]. ROP16, on the other hand, is expressed in genotypes I/III and also plays a key role in the virulence of the parasite [
130]. ROP16 phosphorylates STAT3 and STAT6 [
134], hence downregulating IL-12, which plays a chief role in mounting an immune response against
T. gondii infection [
130]. ROP16 also suppresses T cell activity, hence ensuring immune cell evasion [
135]. Moreover, direct phosphorylation of STAT3 by ROP16 mimics the IL-10 activity and downregulates IFN-γ, hence enhancing the virulence of
T. gondii [
134]. Recently, ROP16-mediated activation of STAT6 proved important for type III
T. gondii survival through suppression of host cell reactive oxygen species production [
136]. Moreover, ROP16 kinase activity silences the
cyclin B1 gene promoter, hijacking the function of the host cell epigenetic machinery [
137]. The role of ROP proteins in the virulence of the parasite makes them excellent drug target candidates to combat toxoplasmosis. A high-throughput screen to identify small molecule inhibitors of ROP18 identified several inhibitors belonging to oxindoles, 6-azaquinazolines, and pyrazolopyridines chemical scaffolds. Treatment of IFN-γ-activated cells with one of these inhibitors enhanced immunity-related GTPase recruitment to wild type parasites [
138]. Thiazolidinone derivatives inhibited
T. gondii in vitro, and in silico analysis demonstrated that the best binding affinity of these derivatives was observed in the active site of kinase proteins with a possible effect of one derivative in the active site of ROP18 [
139] (
Figure 1,
Table 2).