Nuclear envelope lamin A/C type-V intermediate filaments are a major constituent of the mammalian nuclear lamina, a dense fibrous protein meshwork located in the nuclear interior. Lamin A/C proteins control nuclear mechanics and structure and modify cellular signaling, gene transcription, epigenetic regulation, cell cycle progression, cell differentiation, and cell migration. The immune system is constituted by the innate and adaptive immunity. Innate immune response is mediated by myeloid cells such as neutrophils, macrophages, and dendritic cells. These cells produce a rapid and nonspecific response through phagocytosis, cytokine production, and complement activation, as well as activating adaptive immunity. Specific adaptive immune response is provoked by antigen presentation by antigen presenting cells (APCs) and the cytokine milieu, and is mainly mediated by the cellular functions of T cells and the production of antibodies by B cells. Unlike most cell types, immune cells regulate their lamin A/C protein expression relatively rapidly to exert their functions, with expression increasing in macrophages, reducing in neutrophils, and increasing transiently in T cells. In this article, it is discussed and summarized studies that have addressed the regulation of the expression of lamin A/C in cells of the innate and adaptive immune system.
- lamin A/C
- macrophage
- neutrophil
- dendritic cell (DC)
- T cell
- innate immunity
- adaptive immunity
- immune system
- A-type lamins
Nuclear envelope lamin A/C proteins are a major component of the mammalian nuclear lamina, a dense fibrous protein meshwork located in the nuclear interior. Lamin A/C proteins regulate nuclear mechanics and structure and control cellular signaling, gene transcription, epigenetic regulation, cell cycle progression, cell differentiation, and cell migration. The immune system is composed of the innate and adaptive branches. Innate immunity is mediated by myeloid cells such as neutrophils, macrophages, and dendritic cells. These cells produce a rapid and nonspecific response through phagocytosis, cytokine production, and complement activation, as well as activating adaptive immunity. Specific adaptive immunity is activated by antigen presentation by antigen presenting cells (APCs) and the cytokine microenvironment, and is mainly mediated by the cellular functions of T cells and the production of antibodies by B cells. Unlike most cell types, immune cells regulate their lamin A/C protein expression relatively rapidly to exert their functions, with expression increasing in macrophages, reducing in neutrophils, and increasing transiently in T cells.
- Lamin A/C
The mammalian nuclear envelope separates the nucleoplasm from the cytoplasm and is composed of two lipid bilayers: the outer and inner nuclear membranes, nuclear pore complexes, and the nuclear lamina [1]. The outer nuclear membrane (ONM) is continuous with the endoplasmic reticulum, whereas the inner nuclear membrane (INM) surrounds the nuclear lamina [1]. The nuclear lamina is a dense fibrous protein meshwork mainly composed of type V intermediate filament proteins called lamins; these are closely connected to a variety of INM-associated proteins and interact with portions of the chromatin [1]. Lamins can be categorized as A-type or B-type, based on their primary sequence and biological properties [2]. In mammals, lamins are encoded by three genes, i.e., LMNB1 encodes lamin B1, LMNB2 encodes lamin B2 and lamin B3, and LMNA encodes the major forms lamin A and C (referred to as lamin A/C in this manuscript), as well as lamins AΔ10 and C2 [1,3,4].
Lamin A/C contributes to nuclear mechanical stability, nuclear structure maintenance, and nuclear positioning, and mediates higher-order chromatin organization, epigenetic regulation, nuclear pore complex organization, gene transcription, nuclear envelope breakdown, and reassembly during mitosis, DNA replication, DNA damage response, cell cycle progression, cell differentiation, and cell polarization during migration [1,5–7]. These functions have been investigated in diverse cell types, but only a few studies have been performed on immune cells. In this review, we summarize the role of lamin A/C in immune system-mediated cellular mechanisms and its importance in some immune system-associated human diseases.
- Immune System
The immune system is composed of two major arms: innate and adaptive immunity. Innate immunity is mediated by myeloid cells, which generate a rapid and nonspecific response as a first line of defense. Innate immune cells express pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), allowing them to recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Innate immune cells mediate host defense and inflammation by producing cytokines and chemokines, activating the complement cascade and phagocytosis, or activating adaptive immunity by presenting antigens. Notable cells of the innate immunity include neutrophils, macrophages, and dendritic cells (DCs) [8–10].
Specific adaptive immunity is activated by antigen presentation by antigen presenting cells (APCs) and the cytokine microenvironment, and is mainly mediated by the cellular function of CD4 and CD8 T cells and the production of antibodies by B cells. Other cytotoxic cells, such as natural killer T cells (NKT cells) and γδ T cells, are at the border between innate and adaptive immunity [8–10].
- Lamin A/C Expression in Immune Cells
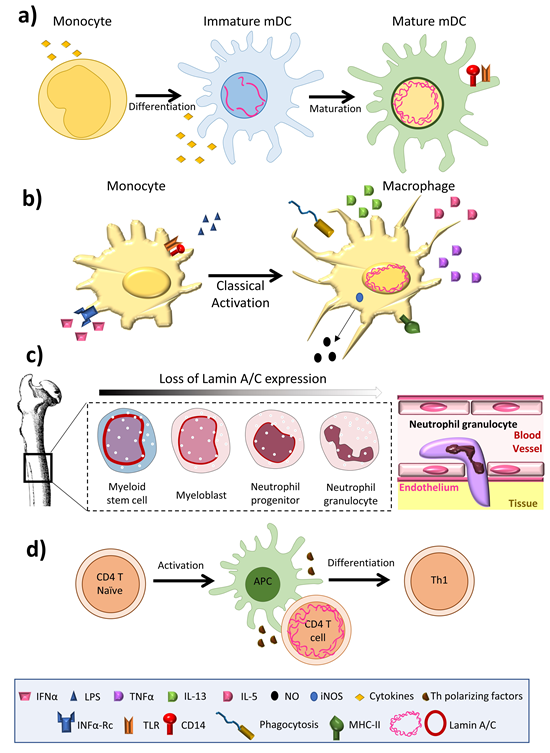
Lamin A/C is abundantly expressed in most differentiated cells, but is absent or infrequently expressed in pluripotent stem cells and embryos during early development [11]. The amount of lamin A/C in interphase of somatic cells is quite stable, exhibiting slow subunit exchange [4]; its expression has thus been linked to cell differentiation [12]. Aging is associated with small changes in the amount of lamin A/C in osteoclasts [13]. The amount of lamin A/C varies greatly between immune cell types, with macrophages and dendritic cells expressing high levels [14,15], but inactivated T and B cells expressing barely detectable amounts [16,17] (Figure 1). Remarkably, unlike most other somatic cells, immune cells have been shown to undergo very rapid changes in lamin A/C protein level during differentiation, activation, or migration [16–19].
Among innate immune cells, high lamin A/C mRNA expression has been reported in human monocyte-derived dendritic cells [20], and high protein expression is observed in rat bone marrow derived dendritic cells [14] (Figure 1a) and macrophages [15,21–23] (Figure 1b). Serum-free differentiation of rat and human macrophages was accompanied by increased expression of lamin A/C [14]. Lamin A/C is also expressed in thyoglycolate-induced mouse peritoneal macrophages and the mouse monocyte/macrophage-like cell line J774A.1 [21,24]. Lamin A/C expression increases during the differentiation of human peripheral-blood monocytes into macrophages [22]. Human promyelocytic leukemia HL-60 cells can be induced to differentiate in vitro into monocytes/macrophages with phorbol ester (TPA) or into granulocytes with retinoic acid. HL-60 cells contain 0.1 × 106 copies of lamin A/C per cell [25]. Phorbol ester-induced HL-60 differentiation to the macrophage phenotype induces lamin A/C expression [25,26] and promotes the redistribution of lamin A to the nuclear periphery [23]. In contrast, differentiation to neutrophil granulocytes is associated with lamin A/C downregulation [27]; human primary neutrophils express very low levels of lamin A/C [28], and retinoic acid differentiation of HL-60 cells into neutrophils results in a downregulation of lamin A/C expression [29,30] (Figure 1c). Macrophage-differentiated HL-60 cells thus contain higher amounts of lamin A/C than neutrophil-type cells [30,31] (Figure 1b,c).
Figure 1. Lamin A/C levels are finely regulated in immune cells. (a) Dendritic cells have an intermediate lamin A/C content, between that of neutrophils and macrophages, which is associated with intermediate viability and migration. (b) Macrophages increase lamin A/C content upon differentiation and activation. (c) During granulopoiesis, neutrophil precursors change their round nuclear shape for a characteristic lobed nucleus, a process linked to almost complete loss of lamin A/C expression and augmented expression of the lamin B receptor (LBR). Neutrophil loss of lamin A/C enables them to pass through narrow spaces. (d) T cells show a transient peak in lamin A/C expression upon recognition of an antigen presented by an antigen presenting cell.
In the adaptive immune system, lamin A/C expression is undetectable in resting B lymphocytes from human blood [17] and mice [32], but increases upon treatment with lipopolysaccharide (LPS) [17]. Lamin A/C has been detected in some B cell lymphomas [17,33], but not in unstimulated human and mouse T lymphocytes [34,35]; it is present in only very few lymphocytes from rat bone marrow cultures [21]. However, lamin A/C has been detected in activated human peripheral blood lymphocytes, CD4+ T lymphocytes, and in CD30+ lymphoid cells [33,36,37]. In line with this evidence, although very few resting human and mouse T cells express lamin A/C, T cell activation by antigen recognition or another TCR-dependent stimulus triggers a transient and potent increase in lamin A/C mRNA and protein expression [16]. In line with this finding, activation of peripheral blood mononuclear cells with the plant lectin concanavalin A results in a sharp increase in the percentage of lamin A/C positive cells [38], and lamin A/C expression is also potentiated during phorbolester-mediated differentiation of HL-60 cells into macrophage-like cells [23].
Little is known about the mechanisms regulating lamin A/C expression in immune cells. One candidate is the Akt/PKB signaling pathway, which is induced in immune cells, for example upon T cell activation [39], and regulates pre-lamin A transcription during interphase in other cell types, possibly via the transcription factors FoxO, Sp1/Sp3, AP1, and CREB [40]. The peak in lamin A/C expression upon T cell activation is followed by a sharp decline [16], which may be the result of diminished de novo synthesis or increased elimination of lamin A/C proteins. Another possibility is that lamin A/C expression in immune cells is controlled by microRNA. Brain expression of lamin A, but not lamin C, is regulated by the microRNA miR-9 [41]. Moreover, a retinoic acid (RA) responsive element has been found in the LMNA promoter of P19 embryonic carcinoma cells [42], and RA reduces lamin A/C expression in HL-60 cells [30] and human monocyte-derived myeloid cells [43]. In T cells, RA binding to RARα or RARγ reduces antigen-recognition-induced lamin A/C expression in CD4+ T cells in vitro [44]. Moreover, the lamin A/C content in CD4+ T cells upon antigen recognition depends on the specific T cell microenvironment in vivo; lamin A/C is highly expressed in activated CD4+ T cells located in peripheral lymph nodes, whereas expression is significantly lower in activated CD4+ T cells in mesenteric lymph nodes and spleen [44]. This effect correlates with known tissue-microenvironment-dependent variations in RA disposal [45]. CD103+ DCs in the gut, mesenteric lymph nodes, and Peyer’s patches can release RA to T cells undergoing activation [46], whereas CD103- DCs, located mainly in peripheral lymph nodes, do not produce RA [47].
- 4. Discussion
Recent advances suggest that lamin A/C is finely regulated in immune cells, in both the level and the time window of expression. Lamin A/C is highly expressed, and the performance of their cell functions requires macrophages to increase its expression, while neutrophils decrease expression. This regulation appears to be related to the specific necessities of these cells types, with increased lamin A/C expression in macrophages potentially related to the exertion of proinflammatory functions and long lifespan, whereas lamin A/C inhibition in neutrophils facilitates migration while reducing lifespan. Lamin A/C expression in DCs seems to be intermediate between macrophages and neutrophils, allowing more efficient and faster migration than macrophages, and longer viability than neutrophils. T cells are a case apart, with transient lamin A/C expression increasing cellular activation and direct differentiation toward a more proinflammatory Th1 phenotype, while decreased expression appears to have no effect on proliferation and probably migration. Overall, increased lamin A/C expression appears to be compatible with higher cell activation, as observed in naïve T cells upon antigen recognition and macrophages upon LPS stimulation. The importance of lamin A/C expression for cell activation and its microenvironment-dependent regulation in activated T cells suggest that this regulation might be reproduced in other immune cell types, pointing to possible future research directions.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21176109