Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Polyphenols are a broad class of molecules extracted from plants and have a large repertoire of biological activities. Biomimetic inspiration from the effects of tea or red wine on the surface of cups or glass lead to the emergence of versatile surface chemistry with polyphenols. Owing to their hydrogen bonding abilities, coordination chemistry with metallic cations and redox properties, polyphenols are able to interact, covalently or not, with a large repertoire of chemical moieties, and can hence be used to modify the surface chemistry of almost all classes of materials.
- polyphenols
- interactions with collagen
- dentin
- enamel
- dental resins
- antibacterial activity
1. Introduction
The entry point for nutrients and food in the body is the oral cavity. Tea and some plants contain natural polyphenols [1]. Polyphenols are secondary metabolites defined by the presence of a minimal number of phenol groups [2,3]. Polyphenols play an interesting role in the oral cavity against many diseases, infections, and oral cancers [4]. This is because they have significant features such as antibacterial activities [5], antioxidant effects [6] in the oral cavity. More recently, many investigations have shown that polyphenols can serve as “processing cofactors” to improve mechanical and functional properties of biomaterials [7]. For the same reasons, they are used in many dental applications. In this study, we focus on the most important polyphenols used in dental applications as tannic acid, catechin, resveratol, gallic acid, and epigallocatechin gallate (Figure 1). Our review is aimed to complete the article written by Catapano-Martinez et al., which described the benefit of polyphenols from food for the dental health [8]. Herein we will not only focus on antibacterial applications but also on the use of polyphenols to improve the adhesion between adhesives and dentin or enamel as well as their role in remineralization processes.
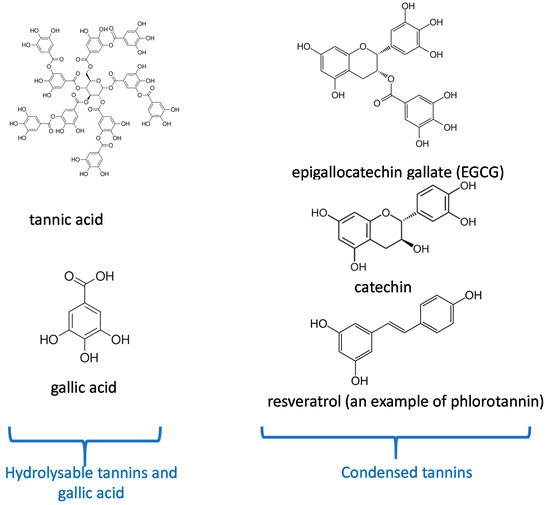
Figure 1. Chemical structure of the most important polyphenols used in dentistry according to their classification [10].
As a starting point, in addition to their structure dependent properties, many polyphenols spontaneously adhere to the surface of almost all known materials [9] allowing to use them in a vast repertoire of materials functionalization strategies. Those possible post-functionalizations rely on the chemical versatility of polyphenols able to undergo acid-base reactions, oxidation processes, chemical reactivity with nucleophiles in the oxidized state, and also chemical coordination with metallic cations [10]. In particular, polyphenols are able to interact with biological and synthetic macromolecules in either a non-covalent or a covalent manner [11]. We build our description of the dental applications of polyphenols based on their well-accepted classification between condensed and hydrolysable polyphenols [10]. Concerning the different subsections describing the application of each class of polyphenols we rely mostly on the anatomy of the teeth and their contact area with saliva concerning their antibacterial activity.
The purpose of this article is to review the literature in which polyphenols were used in dental treatments and engineering. Our literature review relies on PubMed to identify the articles with the following key search sentences: polyphenols from plants and drinking habits in dental caries, polyphenols in enamel, dentin, root canal and, dental pulp treatments, polyphenols in dental adhesives and cements. At purpose, the review is organized with respect to the different applications of polyphenols in the oral cavity and with respect to the big polyphenol families, each one having its chemical and biological specificities [10]. Condensed polyphenols are stable in water but contain a lower fraction of adjacent hydroxyl groups and are often less active for a given chemical interaction than their hydrolysable counterparts, dense in 1,2 diols, but prone to hydrolysis and, hence, fast degradation in water. We will also address the dental applications of polyphenol mixtures. However, it will be apparent that some investigations were devoted to study different kinds of the dental applications, rendering our classification arbitrary in some aspects. We decided to describe the major application of a given investigation in the subsection where the study gave the more pertinent results without neglecting to describe the other applications of the used polyphenol/polyphenol mixture.
2. Condensed Polyphenols
In this section, we will describe the different major dental applications of condensed polyphenols based on flavonoids and on phlorotannins, such as resveratrol, emphasizing on the mechanism of their action as well as on the effective concentration range.
2.1. Dentin Modifier, Dentin Pretreatment, Collagen Cross-Linking, and Resin-Dentin Stability
Dentin is the substance located under the enamel layer. It contains essentially hydroxyapatite crystals and a collagen matrix [12,13]. Collagenase and different protease enzymes (Cysteine cathepsins and matrix metalloproteinases) have been reported to cut collagen chains and are responsible for collagen degradation [14,15]. The adhesion of an adhesive-resin material to dentin depends on the formation and the stability of a hybrid zone (zone where the resin infiltrates into the collagen fibrils of the dentin matrix) that forms a micromechanical interlocking between the resin and the dentin matrix [16]. Therefore, the stabilization of collagen in dentin and in the hybrid layer is possible by preventing the hydrolytic and enzymatic degradation catalyzed by the matrix metalloproteinases and the Cysteine cathepsins.
Proanthocyanidins are secondary class of non-hydrolysable plant metabolites used to pretreat dentin, to enhance its mechanical properties and to reduce the collagen digestion. Thus, proanthocyanidins are a clinical agent for dentin bio-modification [17,18]. The monomeric polyphenolic unit in proanthocyanidins interacts with collagen I, essentially with proline residues, to provide a stable interaction between the resin and dentin for 12 months. The stability of this interaction with time is due to a reduced biodegradation of the dentin matrix with low collagen digestion [19]. Tang et al. [20] showed that the use of 15% “w/v” of grape seed extract (GSE), which contains proanthocyanidins, in contact with demineralized dentin protects the collagen matrix against degradation. They reported that using a solution with 15% of GSE for a 2 min treatment promotes the dentin remineralization rate and forms mostly hydroxyapatite crystals.
A comparison between three polyphenol solutions (at 50 g/L), quercetin, proanthocyanidins and baicalein, showed that the baicalein has the highest effect in the protection of the dentin matrix against collagenase digestion [21].
The formation of polyphenol induced cross-links in the collagen matrix provides cohesion and makes it more resistant to degradation [22].
Liu et al. [23] reported that using 1% “w/w” of grape seed extract ((+)-catechin, (−)-catechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, (−)-epigallocatechin gallate, procyanidin B2, and a pCT-pCT dimer) for 1 min stabilizes the demineralized dentin and provides the cross-linked dentin-collagen complex.
The strong interactions with collagen have necessarily some effect on the mechanical properties of dental tissues: indeed oligomeric proanthocyanidins enhance the elastic modulus of dentin, [24,25]. Concerning the resistance of enamel to dental abrasion was made using mammals’ teeth [26]. Using a hardness test (Vickers indenter), it was shown that saliva containing polyphenol compounds (0.1 M epigallocatechin gallate) in contact with the enamel surfaces showed a greater resistance against abrasion than the enamel put in contact with saliva without added polyphenols.
In addition to the intrinsic mechanical properties of dental tissues, their interaction with other materials, such as resins, needs to be improved. To that aim, dental restorations require tooth surface preparation, this preparation creating a layer of dentinal debris called the “smear layer”. Acid conditioners of dentin are recommended to achieve a clean dentin surface to subsequently provide the required bond strength [27,28,29].
Epigallocatechin-3-gallate (ECGC) solutions with different concentrations (0.02, 0.1, and 0.5% w/v) were used in dentin pretreatment followed by etch-and-rinse adhesive [30]. The dentin treated with a concentration of 0.5% showed a lower bond strength for resins after one day than dentin treated with solutions at the other concentrations. In contrast, dentin treated with all the polyphenol concentrations preserves its bond strength values for resins during 6 months [30]. Accordingly, Singh et al. [31] reported that dentin treated with ECGC at 0.1% w/v preserves the bond strength for 6 months in an etch-and-rinse adhesive system. Contrarily, different concentrations of EGCG (0.02, 0.2 and 0.5% w/v) followed by two-step etch-and-rinse adhesive system had not preserved the bond strength for 1 year of storage in water [32]. These authors reported that the pretreatment with ECGC could reduce the nanoleakage of the resin-dentin interface in time hence reducing the bond strength values. Costa et al. [33] compared the effect of 0.1% of ECGC and 2% w/v of chlorhexidine as dentin pretreatment to a self-etch system. They demonstrated that the ECGC did not affect the bond strength. The dentin having undergone chlorhexidine pretreatment showed lower values of bond strength after 24 h and 6 months of ageing in water. Another study compared the effect of 0.2 M ECGC and 0.2 M catechin as dentin pretreatment for 1 h in self-etch and rinse-and-etch systems [34]. ECGC treated samples revealed a higher bond strength compared with catechin treated samples due maybe to the higher number of hydroxyl groups in EGCG (Figure 1), thus the ability to establish more hydrogen bond interactions.
ECGC enhances the bond strength of fiber post (a direct restorative dental material) bonded with adhesive-resin and cemented with dual-cure composite resin cement to intraradicular dentin which was treated with sodium hypochlorite. In this study, a push-out test was used to evaluate the bond strength. The authors demonstrated that using an ECGC solution at 400 µg/mL for 1 min as the final irrigation in intraradicular dentin, treated with sodium hypochlorite, increased the push-out strength and bond stability of fiber post for a self-etching and an etch-and-rinse adhesive system [35]. Accordingly, in the study of Pheenithicharoenkul et al. [36], the use of a 1 mg/mL ECGC solution (for 10 min) or ethylenediaminetetraacetic acid (EDTA) at 17% w/v (for 5 min), followed by ECGC (for 5 min) as the final canal irrigation, demonstrated a higher bond strength than the use of EDTA alone or EDTA with sodium hypochlorite without ECGC in the final canal irrigation solution.
Bonding the dental restoration to the structure of the tooth becomes a routine in dental practice and requires various properties, such as a good sealing, high bonding strength to tooth surfaces, durability over time, low toxicity, and low degradation rate [37,38,39].
Epigallocatechin-3-gallate (EGCG) is a polyphenol which has antibacterial and antioxidant activities [40,41] as will be described in Section 2.3. In addition to those properties, EGCG was incorporated in dental adhesive-resins for its inhibitory effects of matrix-metalloproteinases (MMPs) and cysteine cathepsins. Indeed, those proteins are major players in the degradation and perturbation of the resin-dentin interface [42,43].
At concentrations of 0.5% and 1% (w/v), EGCG incorporated in an adhesive resin could increase the bond strength values and the longevity of adhesive-dentin bond for 6–12 months [44,45]. In contrast to the previous investigation, the addition of 0.01 and 0.1% (w/v) of EGCG to adhesive-resin compounds did not affect the resin-dentin bond, but could reduce the solubility of the adhesive-resin in water [42]. Yu et al. [46] showed that EGCG, in addition to its antibacterial activity, increases the bond strength of root canal sealer to dentin. This result was obtained using a push-out test after a thermocycling procedure (5000 cycles).
The dental adhesive-resin incorporates many toxic compounds such as Bis-GMA, TEGDMA, and Bis-phenol A [47]. Fonseca et al. [43] demonstrated that the presence of 0.5–1% (w/w) of EGCG in the adhesive-resin could reduce the toxicity, reduce the solubility and the water sorption of this adhesive. These concentrations (0.5–1%) could maintain the hybrid layer and preserve the bond strength over time.
Glass ionomer cements were also blended with 0.1% (w/w) EGCG to improve their antibacterial activity and to increase their mechanical properties such as the flexural strength and the hardness [48].
Dental adhesive-resins were also modified with quercetin, which is known as an amphiphilic antioxidant. The addition of 500 µg/mL quercetin to dental adhesive could give an antibacterial effect and preserve its bond strength by inhibiting the collagenase activity [49].
Gotti et al. [50] analyzed the effect of the addition of 5% (w/w) quercetin to a two-step etch-and-rinse, two-step self-etch and one step self-etch adhesive system on bond strength durability to dentin surfaces after two storage periods in water (24 h and 6 months). They demonstrated a negative effect on the bond strength of these adhesives after 24 h. In contrast, for a 6 months storage period, the two-step etch-and-rinse and the two-step self-etch adhesive systems incorporating quercetin increased the bond strength, whilst the one step self-etch adhesive incorporated with quercetin maintained the bond strength durability.
2.2. Remineralization, Cell Viability, and Differentiation
ECGC and epicatechin gallate (ECG) (10 mmol/L) did not affect the dental pulp cells viability [51].
Similarly, Lim et al. [52] reported that epicatechin (0.01, 0.05, or 0.1 mM) as a collagen cross-linker did not affect the cell viability and induced a positive effect on the proliferation and differentiation of human dental pulp cells.
Three flavonoids (quercetin, genistein, and baicalin) and phenamil -an osteoblast differentiation molecule- were used to test their cytotoxicity and their osteoblast differentiation activity on dental human cells [53]. The used concentrations, ranging from 1 to 25 µM, did not alter the cell viability. Among the three tested polyphenols, phenamil had the strongest influence on alkaline phosphatase activity. In contrast, this in vitro study demonstrated that quercetin had a superior effect to phenamil in the osteogenic differentiation.
Osteoclasts and osteoblasts play an important role in orthodontic tooth movements. These movements could produce complications, such as root resorption. Liu et al. [54] used two doses of resveratrol (5 mg/kg/day and 10 mg/kg/day) to evaluate its effect on the orthodontic tooth movement and root resorption. This study, performed on rats, demonstrated that resveratrol could inhibit tooth movement during orthodontic treatment and reduce the rate of root resorption during orthodontic therapy as described in (Figure 2). This investigation also showed that resveratrol promotes the osteoblastic activity and reduces the osteoclastic activity during orthodontic therapy. Therefore, resveratrol application during orthodontic therapy could be used as a novel approach to prevent undesired tooth movement (anchorage or relapse).
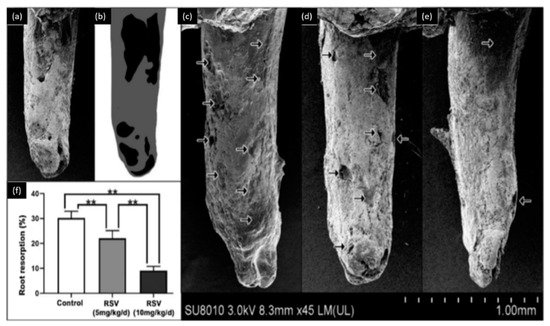
Figure 2. Observation of root resorption. (a) SEM image of the tooth, (b) root resorption area (black) and the total surface area (grey), (c) representative SEM pictures of the control group, (d) 5 mg/kg/d resveratrol group, (e) 10 mg/kg/d resveratrol group, (f) the resorption ratio of the three groups were calculated by dividing the surface of black area by the grey area. (** p < 0.01, n = 6). Modified from ref. [54] with authorization.
Tooth development may be perturbed during exposure to high-energy radiations. Barbosa et al. [55] used resveratrol, as a radioprotector, on rats to analyze its effect. Each rat received 100 mg/kg of resveratrol. Barbosa et al. concluded that this dose of resveratrol had no radioprotective effect on the dental tooth structure.
2.3. Antibacterial Activity
It has first to be emphasized that the antibacterial activity of polyphenols is related to a combination of mechanisms: implying the inhibition of enzymes implied in the bacterial metabolism as well as the change in the redox balance in the bacterial cell (owing the possible oxidation of catechol groups in quinones) and the reduction in the concentration of metallic cations due to complexation by polyphenolics [10].
Xu et al. [56] showed that a concentration of 31.25 µg/mL of epigallocatechin gallate from green tea inhibits the glucosyltransferase activity of Streptococcus mutans and 15.6 µg/mL of the same molecule inhibits 90% of S. mutans biofilm formation.
Among condensed polyphenols contained in tea, (−)-epigallocatechin gallate (ECGC) binds to alpha-amylase, which is a salivary enzyme that catalyzes the breakdown of starch. ECGC could inhibit the activity of alpha-amylase by non-competitive inhibition. The antimicrobial activity of EGCG against Aggregatibacter actinomycetemcomitans was showed at concentrations higher than 0.5 mg/mL [57].
In the study of Feng et al. [58], the most abundant polyphenols isolated from green tea were catechin and 1,4,6-tri-O-galloyl-βD-glucose. Catechin derivatives as gallocatechin gallate (0.32 mM) and epigallocatechin gallate (0.31 mM) were able to inhibit S. mutans glucosyltransferases.
Melok et al. [59] showed that the use of 250 µg/mL of epigallocatechin-3-gallate- stearate, which is an esterified derivative of epigallocatechin-3-gallate could completely inhibit S. mutans growth and biofilm formation. Using scanning electron microscopy, they evidenced the antibacterial effect of epigallocatechin-3-gallate-stearate after four days of treatment (Figure 3).
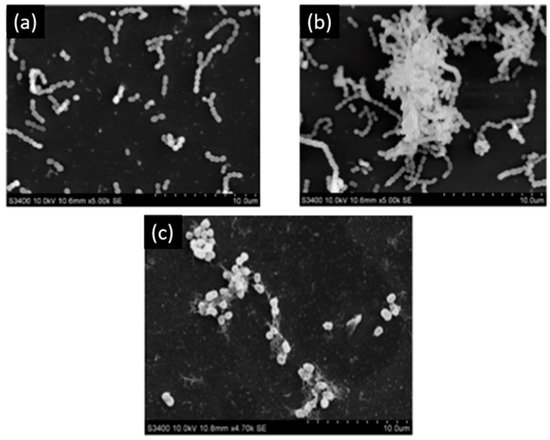
Figure 3. Scanning electron microscopy of S. mutans. (a) Control; (b) untreated S. mutans cells after 4 days; (c) S. mutans cells treated with 250 µg/mL of epigallocatechin-3-gallate-stearate after 4 days. Modified from Ref. [59] with authorization.
ECGC was used as an antibacterial agent against Enterococcus faecalis biofilms, which are associated with persistent root canal infections [60]. The minimum inhibitory concentration of ECGC against E. faecalis was equal to 5 µg/mL. Lee et al. reported that a concentration of 500 µg/mL applied for 7 days completely eradicated the E. faecalis biofilm [60].
EGCG was added to dental restorative composites to afford this material with an antibacterial activity [61]. The addition of 700 µg/mL of EGCG reduces the viability of S. mutans.
To provide some comparative data, Kwon et al. [62] compared the effects of two antibacterial agents and of two cross-linkers (ECGC and glutaraldehyde) on cell viability, odontogenic differentiation, and proliferation of dental pulp cells, and antibacterial activity in collagen scaffolds. The results revealed that the cell viability is reduced in the presence of glutaraldehyde (0.1, 1, 10, and 100 µmol/L) compared with the presence of ECGC (0.1, 1, 10, and 100 µmol/L). In the presence of the highest ECGC concentrations (10–100 µmol/L), the cell viability was nevertheless reduced. It was also concluded that ECGC did not promote the odontogenic differentiation and proliferation by itself but facilitated these processes. The cross-linked collagen produced in the presence of ECGC showed a shorter setting time, a higher compressive strength and a rougher surface. Equal concentrations of ECGC and glutaraldehyde reduced the growth of S. mutans.
Another study showed that 200 µg/mL and 300 µg/mL of EGCG have an inhibitory effect on the growth of S. mutans (Figure 4) and increase the bond strength of the resin to dentin surface. EGCG also preserves the durability of the bond strength for six months [63]. Hence, this investigation is prototypal of the multi-functionality of EGCG in dental applications.
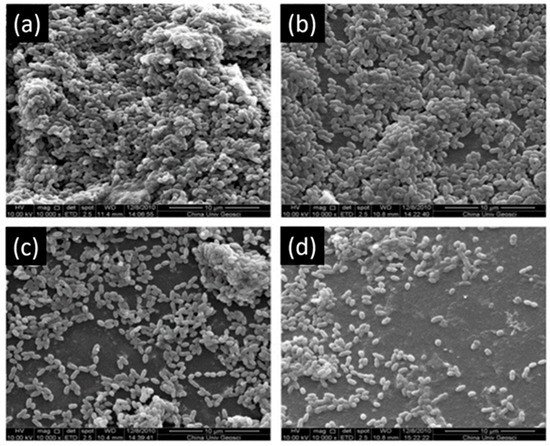
Figure 4. Scanning electron microscopy of S. mutans accumulation on specimens. (a) Adhesive resin surface without epigallocatechin-3-gallate (ECGC); (b) ECGC 100 µg/mL; (c) ECGC 200 µg/mL, (d) ECGC 300 µg/mL. Biofilms accumulated on (c) and (d) were not compact after 24 h incubation. Modified from ref. [63] with authorization.
2.4. Anti-Inflammatory and Antioxidant Activity
Methacrylate resin-based materials contain triethylene glycol dimethacrylate (TEGDMA), which induces the expression of cyclooxydenase-2. This TEGDMA-induced cyclooxydenase-2 plays a role in dental pulp diseases and pulpit. The cyclooxydenase-2 activity could be suppressed in the presence of 10 and 15 µmol/L of ECGC [64].
Catechins, including epicatechin gallate and epigallocatechin gallate were used to reduce and inhibit the inflammatory factors, which are found in inflamed pulp [65]. In the study of Hirao et al., two catechin concentrations were used (10 and 50 µg/mL) to show that this polyphenol did not affect the viability of the human dental pulp fibroblast cells. Hence, catechins inhibited the effect of interleukins (IL-8 and -6), of monocyte-chemoattractant proteins and of prostaglandins which are receptors stimulating the pro-inflammatory mediators. Contrarily, high concentrations of epigallocatechin gallate (50 µg/mL) did not inhibit the production of prostaglandins in pathogen-associated molecular patterns simulated human dental pulp fibroblasts. Therefore, catechin has an anti-inflammatory effect due to its activity on the inhibition of the cytokines and chemokines in human dental pulp fibroblasts modified with caries-related S. mutans, Streptococcus sanguinis and Streptococcus salivarius, and pathogen-associated molecular patterns. Accordingly, Nakanishi et al. [66] showed the benefits of using epicatechin gallate (ECG) and epigallocatechin gallate (ECGC) to inhibit the expression of pro-inflammatory cytokines and adhesion molecules in human dental pulp cells.
Similarly, EGCG and ECG (dissolved at 20 and 50 ug/mL) reduced the up-regulated expression of vascular endothelial growth factors and cyclooxygenase-2 which are induced pro-inflammatory cytokines in dental pulp cells simulated with lipopolysaccharide (LPS), peptidoglycan (PG), interleukin-1b (IL-1b), or tumor necrosis factor-α (TNF-α) [67].
Wang et al. [51] also demonstrated that the anti-inflammatory activity of ECG and ECGC proceeds through the inhibition of the activity of nuclear factor-kappa B (NF-κB).
Reactive oxygen species (ROS) caused by pulp diseases and dental bleaching agents generate oxidative stress [68]. Park et al. [69] reported the effect of ECGC (5–50 µM) against nitric oxide-induced toxicity of human dental pulp cells which is driven by the ROS production. The Bcl-2 cell family contains anti- and pro-apoptotic proteins, which are important moderators in regulating cell death. In this context, ECGC scavenges the ROS and regulates the expression of the Bcl-2 family preventing the nitric oxide-induced apoptosis.
ROS can also be quenched by butein, which is a plant polyphenol and one of the most active compounds of the Rhus Verniciflua plant found in East Asian countries [68]. The study of Lee at al. demonstrated that butein quenches the ROS and suppresses the toxic effects of hydrogen peroxide, which is used as a bleaching agent. The concentrations of butein (2.5–20 µM) had no toxic effect on the dental pulp viability. The maximal heme oxygenase-1 protein expression and heme activity, which exhibit many cytoprotective effects and remove pro-oxidant heme molecules, were attained after 18 h of butein exposure to human dental cells. Nuclear accumulation of nuclear factor-E2 caused by butein treatment increased the promotor activity of antioxidant response elements. Therefore, butein can prevent functional dental cell death and could be used as a protective agent in dental pulp diseases.
Mahmoud Hashemi et al. [70] evaluated the effect of adding 0.5 mg/mL of quercetin to simulated T cells extracted from pulpits with high mobility group box 1 (HMGB1). They demonstrated that quercetin can decrease pro-inflammatory cytokines such as interleukin-6 and -1β with blocking high mobility group box 1 and inhibiting the mitogen activated protein kinase (MAPK) signaling pathway.
Luteolin (used at 35 µmol/L) is also a polyphenol from the flavonol family and was combined with phosphorylated pullulan to decrease the production of inflammatory cytokines [71]. It was reported that the combination of both compounds was less efficient than luteolin alone. This may be due to some strong interactions between both compounds reducing the concentration of available polyphenol.
Concerning resveratrol, a member of the phlorotannin subclass of condensed polyphenols (Figure 1), it was shown to inhibit interleukin (IL-8 and -6) and suppresses the c-Jun N-terminal Kinase (JNK) signaling pathway in dental pulp cells simulated by tumor necrosis factor α (TNFα). TNFα is one of the cytokines that initiates the natural inflammatory response in the dental pulp. In contrast, resveratrol did not inhibit the degradation of IκBα nor the phosphorylation and nuclear translocation of p65 NF-κB in TNFα treatments. The results of this study allowed to hypothesize that resveratrol can be beneficial to decrease pulpal damage during the severe phase of inflammation in vital pulp [72]. Another study reported that using resveratrol at a concentration up to 50 µM had no toxic effect on dental pulp stem cells [73]. Resveratrol raises the activity of Sirtuin 1 (stress-activated nicotinamide adenine dinucleotide-dependent protein deacetylase), which is a mediator of the immune and defense genes in human dental pulp cells [74]. Resveratrol (at 5 µmol/L) activates the function of Sirtiun 1, which can promote the osteogenic differentiation of dental pulp stem cells in inflammation microenvironment through Wnt/β-catenin signal [75].
3. Hydrolysable Tannins and Gallic Acid
3.1. Dentin Modifier, Dentin Pretreatment, Collagen Cross-Linking, and Resin-Dentin Stability
Four dentin bio-modifiers extracted from different plants were compared such as hydrolysable tannins from Aroeira, condensed tannins from grape seed with cardol and cardanol from cashew nut shell liquid. The results demonstrated that the four groups achieved cross-linking in dentin matrix after 1 min of treatment and the best bio-modifiers were cardol and cardanol [78].
Tannic acid forms stable cross-links with exposed collagen fibrils allowing to increase the resistance against their degradation process [79]. Bedran-Russo et al. [22] reported that solutions containing 10% and 20% (w/v) of tannic acid could increase the stiffness of demineralized dentin and reduce the enzymatic degradation of collagen most probably due to hydrogen bonds between the biopolymer and tannic acid (TA).
A successful dental root canal treatment depends on various factors such as proper cleaning, and tridimensional filling of the root canal system. The removal of the smear layer and the disinfection of the root canal system is of prime importance during the endodontic therapy [80,81].
Bitter [82] analyzed the effect of using hydrogen peroxide and sodium hypochlorite followed by a solution containing 25% (w/v) of tannic acid as the final irrigation solution in dental root canal. This study showed that using tannic acid in the final irrigation fluid revealed a smoother and cleaner pulp chamber surface compared with hydrogen peroxide and sodium hypochlorite treatment without tannic acid as final irrigation solution. In addition, Bitter [83] showed that using a 25% (w/v) solution of tannic acid in contact for 60 s removed the smear layer without broadening the orifice of dentinal tubules, and removed partially the organic material of the dentinal tubules.
The same group reported that dentin treated with tannic acid had an improved resistance to collagenase degradation [84]. In contrast, another study demonstrated that the application of solutions containing 15, 20, and 25% (w/v) of tannic acid on dentin surfaces for 5, 10, or 15 min could not totally remove the smear layer owing to the astringent action of tannic acid. They reported that tannic acid itself attached to collagen by means of hydrogen bonds [85]. Different concentrations of tannic acid (2, 5, 10, 15, 20, and 25% (w/v)) were applied on dentin surfaces for different times (15, 30 and 60 s) [86]. These researchers reported that a low concentration of tannic acid (2 or 5% (w/v)) applied for 60 s could remove the smear layer, leaving the orifices of dentinal tubules occluded. The dentinal surface that was treated with 20% or 25% (w/v) of tannic acid for 15s revealed incomplete removal of the smear layer and exposure of some of the dentinal tubules. Accordingly, Bitter [84] tested the permeability of methylene blue in 62 dentinal cavities treated with solutions containing 25% (w/v) of tannic acid for 15 s. Their results showed that 48 over 62 cavities did not allow for the penetration of methylene blue into the dentinal tubules.
A glass ionomer was blended with 1.5%, 5%, and 10% (w/w) tannin-fluoride preparation; the 1.5% preparation increased the bond strength of glass ionomer to dentin after one day. The bond strength of glass ionomer modified with tannin-fluoride preparation did not reveal a significant difference after one month [87].
Tannic acid incorporated in polycarboxylate cement, as well zinc fluoride, enhances the resistance of dentinal collagen to collagenase and proteolytic enzymes [88].
The dentin fluid flow is the main cause of dentin hypersensitivity, which was reduced by using gallic acid/Fe+3 complexes (aqueous solutions of FeCl3 (1.2 × 10−3 M) and gallic acid (0.47 × 10−3 M)), which performed for four repeated treatments each lasting over 60 s [89]. This kind of catechol-iron complex is able to deposit on the surface of all known materials [90]. Oh et al. [89] showed that the pyrogallol group of gallic acid binds to dentin and Fe+3 ions create stable cross-linked complexes in an aqueous environment. Their scanning electron microscopy observations showed that dentin treated with gallic acid/Fe+3 complexes create tight bridge like connections between adjacent peritubular dentin, which resulted in less outward flow. Another complex that is fluoride-tannin acid-lanthanum-apatite was used to reduce the dentinal hypersensitivity [79]. The surfaces of the treated samples were completely covered with fine spherical compounds, and the dentinal tubules were occluded with materials (Figure 5).
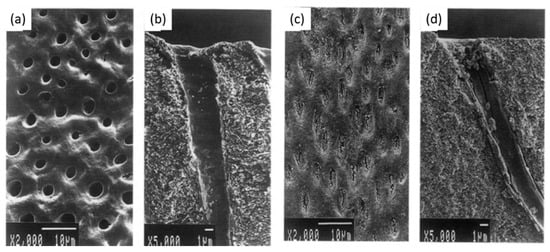
Figure 5. (a,b) Scanning electron micrographs of dentin hypersensitivity, (c,d) scanning electron micrographs of dentin treated with fluoride-tannin acid-lanthanum-apatite. Modified from ref. [79] with authorization.
Three different antioxidants (solutions containing 10% gallic acid, 10% tannic acid, and 10% ascorbic acid “w/v”) were used to irrigate the root canal and to evaluate the infiltration of resin sealer in dentinal tubules [91]. The application of gallic acid for 10 min showed the best penetration of resin sealer into dentinal tubules, maybe due to the presence of three vicinal hydroxyl groups in its structure (Figure 1).
Gallic acid, a polyphenol made from a single aromatic ring (Figure 1) improved its inhibitory effect on matrix metalloproteinases and cysteine cathepsins to improve the durability of bond strength [18]. In contrast, it reduced the mechanical properties of adhesive-resin such as biaxial flexural strength and hardness values [92].
3.2. Remineralization, Cell Viability, and Differentiation
The structure of enamel is an essential portion of the tooth, which is exposed in the oral cavity. It is the hardest and most mineralized tissue of the body [93,94]. Salivary pellicles composed of adsorbed macromolecular compounds delivered from saliva, blood, gingival fluids, bacteria, molecules and particles from the diet [95], Streptococcus mutans (S. mutans) and other oral bacteria adhere on the surface of enamel. All these substances and particles demineralize this surface resulting in the formation of dental caries [96,97,98]. To reduce the severity of such processes, gallic acid, present in various food and plants can inhibit the enamel demineralization as a calcium chelator and can enhance the remineralization of the demineralized enamel. Thus, gallic acid is a promising agent for enamel remineralization and caries treatments [99,100]. Gallic acid was also used to re-mineralize the early carious enamel, to increase the surface microhardness and simultaneously to reduce the wear resistance of enamel [101].
Enamel is mostly composed of mineral, approximately 96% in weight. Owing to its strong chelation ability with Ca2+ cations, gallic acid (GA) was previously used to enhance the remineralization of demineralized enamel [99,100]. Gallic acid (4 g/L) was used to induce the formation of hydroxyapatite (HAP) [102]. It was shown that gallic acid participates in hydroxyapatite formation, limits the crystal growth mainly along the [002] direction and changes the crystal morphology and size. GA-HAP crystals were smaller than the HAP crystals obtained in the absence of polyphenol. The crystal morphology was observed by scanning electron microscopy (Figure 6). It was found that GA-HAP had an urchin-like shape, while loose needle-like crystals were found in HAP formed without additive.
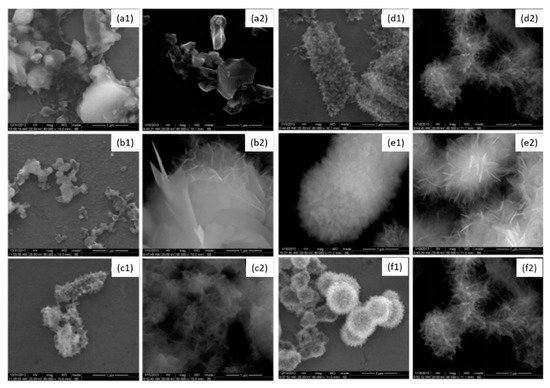
Figure 6. Morphology of hydroxyapatite (1) and hydroxyapatite + gallic acid (2) crystals at different times; (a) = 3 h, (b) = 12 h, (c) = 24 h, (d) = 3 days, (e) = 7 days, (f) = 14 days at 80,000× magnification. Modified from ref. [102] with authorization.
Accordingly, the same authors [103] investigated the morphology and the size changes of crystals as function of the GA concentration (0.05 to 4 g/L). They reported that increasing the concentration in GA reduced the crystal size from 40 to 25 nm. This is the result of gallic acid adsorption on specific crystal faces inhibiting further crystal growth.
3.3. Antibacterial Activity
Apacaries gel is a material containing polyphenols from mangosteen extracts and papain as an enzyme [104]. This gel, including polyphenolic compounds, such as gallic acid, which have antibacterial effects, could play an interesting role in the removal of various tissues.
3.4. Anti-Inflammatory and Antioxidant Activity
In addition to its potent Ca2+ chelator activity, gallic acid is a very active phenolic acid with a high free radical scavenging activity [91].
Surprisingly, it appears that the antibacterial and anti-inflammatory activities of hydrolysable polyphenols were less investigated in the dental field than for their condensed counterparts. This may well be due to their instability in aqueous solutions.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering7030072
This entry is offline, you can click here to edit this entry!
