Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Quinoa is a strategic crop due to its high N content and its adaptability to adverse conditions, where most of the soils are deficient of nitrogen (N).
- quinoa
- nitrogen harvested by yield
- apparent use efficiency of N
- arid environments
- Altiplano
1. Introduction
Integrated nutrient management for food production is an approach and paradigm that supports the food security, conservation, and sustainability of renewable natural resources [1]. Understanding nutrient cycles is essential for improving crop nutritional management. Particularly, in highland and arid agroecosystems such as the southern Bolivian Altiplano, nitrogen (N) supply limits plant growth and development [2]. No other element for life, such as nitrogen, takes so many chemical forms in the atmosphere, soil, and plants [3]. In the atmosphere, the most reactive are N and gas (N2), while in soil, nitrogen oxide, NO, and nitrogen dioxide (NO2) prevail; when fertilizer is used, forms such as ammonia (NH3) can be found; while in water, nitrogen can be present in inorganic forms such as ammonia, ammonium, nitrate, and nitrite, and the organic form is present in proteins, amino acids, urea, and living or dead organisms [4].
In semi-arid and arid land regions, water resources are limited and have significant consequences on the soil nitrogen content [4]. The seasonal distribution of rainfall can affect the accumulation and emission of N in soils during the dry season [5][6]. Nitrogen is accumulated in the soil as wet and dry, and part of it is released to the atmosphere when pore spaces in the soil are filled with water, but this process depends on the soil type and climate [7].
Nitrogen use efficiency (NUE) determination in fragile soils such as the southern Bolivian Altiplano is significant for understanding soil NO3–N converted into grain for quinoa (Chenopodium quinoa Wild), a rainfed crop. NUE can be expressed in several ways: grain production by unit of available N, or index of utilization, which is the absolute quantity of produced biomass per unit of available N [8]. The factors that influence this efficiency are edaphic structure, climatic conditions, interactions between soil and bacterial processes, nature of organic and inorganic nitrogen sources, and availability of N in the soil [9][10]. NUE denotes the relationships between total input compared to the nitrogen output. This is complex and involves absorption, metabolism, and redistribution in the plant. However, adopting a complete crop nutrition strategy allows efficiency, profitability, and sustainability to improve. NUE is a determined metric used to measure N management in the soil [11]. Moreover, NUE is the maximum economic yield produced per unit of N applied, absorbed, or utilized by the plant to produce grain and straw [12]. NUE is partitioned in two processes: (a) absorption efficiency, when the plant is able to remove the available N from the soil usually present as nitrate or ammonium ions, and (b) utilization efficiency, when the plant is able to transfer the available N to the grain as protein [13]. The absorption efficiency is of the utmost importance for predicting plant performance and yield. Most plants capture inorganic N as dissolved nitrate (NO3−) or ammonium (NH4+) from the soil through their roots [14]. Root architecture, morphology, rate of respiration, and transporter activity for available forms of N in the rhizosphere determine N uptake rate. The utilization efficiency requires the process of carbon fixation for nitrogen taken up, photosynthesis, canopy formation, and nutrient remobilization from all tissues to grain during seed filling [10]. The process is initiated once N is introduced into the plant cell and is reduced into organic molecules.
Quinoa is a strategic partner crop for food security as a plant-based protein source [15], and its adaptability to unfavorable growing conditions [16][17]. Quinoa is an Amarantaceae with an intermediate protein content, less than that of legumes and more than that of cereals [17]. The protein content in grain depends on the varieties and soil conditions, and it can reach up to 23%. This protein level requires a significant supply of nitrogen, which is not only essential for the grain, but also for plant growth and development. Its exceptional adaptations to limiting factors in the environment are tolerance to drought [18] and frost [19], as well as to saline and/or low-fertility soils, maintaining adequate yields [20][21]. The Intersalar region, in the southern part of the Bolivian Altiplano has an extreme climate, with rainfall from 150 to 300 mm per year, 200 days of night frost, strong winds, and intense solar radiation [22][23][24]. Furthermore, it has serious degradation problems and low nitrogen levels in the soil. Soil degradation is attributed to monoculture, the use of virgin soils to expand the agricultural frontier, the use of inadequate agricultural machinery (disc plough) in highly susceptible soils to wind erosion, traditional and manual harvesting, the use of left-harvested plants after grain threshing in camelid cattle, llama (Lama glama) or sheep (Ovis aries) feeding, neglect of traditional sowing in the traditional system of sectoral fallowing known as “mantos” [25], the practice of soil fallowing (two to three years without agriculture), and the lack of organic matter due to little or no incorporation of manure (reduction of llama and sheep livestock) and stubble leftover.
In the Intersalar, it has been observed that there are plots with more than 80 agricultural years of production under quinoa monoculture. There are no contributions of manure or other nitrogen mineral fertilizer sources, and the only form of cultural management is the practice of soil fallow (one to two years) [26]. However, acceptable and economically sustainable productivity is still utilized by farmers, and the yields are between 450 and 750 kg ha−1, despite no application of nitrogen [27][28][29][30]. Due to their origin, and as a consequence of the abovementioned factors, the soils of the Intersalar zone are poor in N. Of these soils, 98% are classified as very low in N, while the remaining soils (2%) are classified as low [26][31]. With this position, we ask ourselves, how can quinoa be produced in the Bolivian Altiplano under low levels of nitrogen in the soil? This question was unraveled based on different factors: (1) the effect of fertilization on productivity under rainfed and irrigated agricultural conditions, (2) the top and bottom limits of fertilization, (3) the parameters related to the uptake and assimilation of N, and (4) the effect of monoculture on yield under rainfed agricultural conditions.
2. The Effect of Fertilization on Productivity in Irrigated and Rainfed Cultivation
The average data for each dose of fertilizer presented in Table 1 were used to determine the nitrogen uptake, expressed as kilograms of N to produce one ton of grain, as described in Figure 1. The relationship between nitrogen use efficiency and nitrogen fertilizer rate was remarkably consistent (R2 = 0.88, p = 2.2 × 10−4).
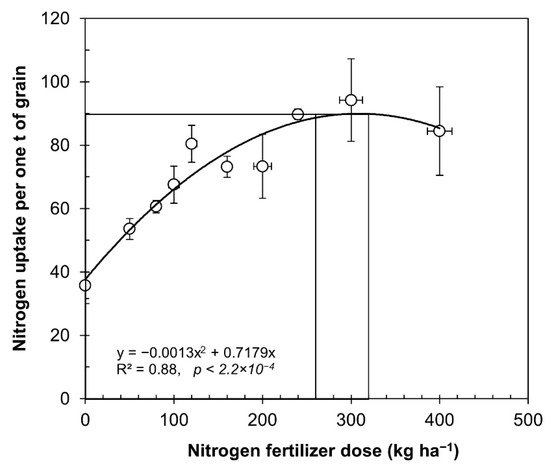
Figure 1. Relationship between nitrogen rates applied and uptake of nitrogen from experiments under irrigated and rainfed conditions. The data from Table 1 were utilized to calculate variations in seed yield in cultivated quinoa under rainfed and irrigated conditions. The symbols represent the average values of equal doses (mean ± SE).
Table 1. Nitrogen dose and yield of grain in various quinoa cultivars under different growing conditions and soil textures.
| Dose (kg ha−1) |
Seed Yield (kg ha−1) | Cultivar | Soil Texture | Irrigation Type | Reference |
|---|---|---|---|---|---|
| 0 | 1166 | Blanca Junin | Sandy clay loam–sandy loam | Rainfed | Borda, 2018 [32] |
| 0 | 1100 | Regalona Baer | Silty clay | Rainfed | Campillo and Contreras, 2019 [33] |
| 40 | 2093 | KVL 8401 | Clay loam | Rainfed | Jacobsen et al., 1994 [34] |
| 80 | 2428 | KVL 8401 | Clay loam | Rainfed | Jacobsen et al., 1994 [34] |
| 80 | 2140 | Regalona Baer | Silty clay | Rainfed | Campillo and Contreras, 2019 [33] |
| 120 | 3500 | Cochabamba y Faro | Clay loam | Rainfed | Schulte et al., 2005 [35] |
| 120 | 2685 | KVL 8401 | Clay loam | Rainfed | Jacobsen et al., 1994 [34] |
| 160 | 2760 | KVL 8401 | Clay loam | Rainfed | Jacobsen et al., 1994 [34] |
| 160 | 3000 | Regalona Baer | Silty clay | Rainfed | Campillo and Contreras, 2019 [33] |
| 240 | 3360 | Regalona Baer | Silty clay | Rainfed | Campillo and Contreras, 2019 [33] |
| 320 | 3540 | Regalona Baer | Silty clay | Rainfed | Campillo and Contreras, 2019 [33] |
| 400 | 3430 | Regalona Baer | Silty clay | Rainfed | Campillo and Contreras, 2019 [33] |
| 0 | 1068 | Faro and UdeC10 | Loam–silty loam | Supplementary | Berti el al., 2000 [36] |
| 0 | 1700 | Altiplano INIA, Salcedo INIA | Sandy loam | Supplementary | Mendoza Nieto et al., 2016 [37] |
| 0 | 1868 | Blanca Real | Sandy loam | Dripping | Llaca, 2014 [38] |
| 0 | 981 | Genotipo O3 | Loamy | Surface | Franco, 2018 [39] |
| 50 | 1848 | Genotipo O3 | Loamy | Surface | Franco, 2018 [39] |
| 75 | 2112 | Faro and UdeC10 | Loam–silty loam | Supplementary | Berti et al., 2000 [36] |
| 80 | 2240 | Blanca Real | Sandy loam | Dripping | Llaca, 2014 [38] |
| 100 | 2700 | Altiplano INIA, Salcedo INIA | Sandy loam | Supplementary | Mendoza Nieto et al., 2016 [37] |
| 100 | 2267 | Genotipo O3 | Loamy | Surface | Franco, 2018 [39] |
| 120 | 3300 | Titicaca | Sandy loam | Deficit irrigation | Razzaghi et al., 2012 [20] |
| 120 | 3000 | Titicaca | Sandy clay loam | Deficit irrigation | Razzaghi et al., 2012 [20] |
| 120 | 2300 | Titicaca | Sandy | Deficit irrigation | Razzaghi et al., 2012 [20] |
| 150 | 2456 | Faro and UdeC10 | Loam–silty loam | Supplementary | Berti et al., 2000 [36] |
| 150 | 2541 | Genotipo O3 | Loamy | Surface | Franco, 2018 [39] |
| 180 | 3413 | Salcedo INIA | Sandy loam | Dripping | Herreros, 2018 [40] |
| 200 | 2800 | Altiplano INIA, Salcedo INIA | Sandy loam | Supplementary | Mendoza Nieto et al., 2016 [37] |
| 200 | 1659 | Genotipo O3 | Loamy | Surface | Franco, 2018 [39] |
| 225 | 2912 | Faro and UdeC10 | Loam–silty loam | Supplementary | Berti et al., 2000 [36] |
| 240 | 3240 | Blanca Real | Sandy loam | Dripping | Llaca, 2014 [38] |
| 270 | 4249 | SalcedoINIA | Sandy loam | Dripping | Herreros, 2018 [40] |
| 300 | 2600 | Altiplano INIA, Salcedo INIA | Sandy loam | Supplementary | Mendoza Nieto et al., 2016 [37] |
| 360 | 3783 | Salcedo INIA | Sandy loam | Dripping | Herreros, 2018 [40] |
| 400 | 2100 | Altiplano INIA, Salcedo INIA | Sandy loam | Supplementary | Mendoza Nieto et al., 2016 [37] |
From the data obtained, we can infer a normal curve, with an increase in nitrogen uptake per ton of produced grain, up to applications of 260 kg ha−1, after which it started to become asymptotic, and over 400 kg ha−1, the yields began to decrease as further nitrogen fertilizers were incorporated. In Figure 1, researchers demonstrate that nitrogen uptake increased when the nitrogen fertilizer rate increased from 35 kg of N per ton of with no nitrogen fertilizer, until reaching the optimum 90 kg of N per ton of produced grain with 260 kg N ha−1. Alvar-Beltran et al. used three doses of nitrogen fertilization (25, 50, and 100 kg N ha−1) and the extraction was 25 kg N per ton of grain produced (1:40 ratio) [41].
2.2. The Limits of Fertilization in Quinoa
Table 2 shows the efficiency indicators based on the average yields from each nitrogen fertilization rate, according to the data and average values in Table 1.
Table 2. Efficiency indicators according to various fertilization tests under irrigated and rainfed conditions.
| Nitrogen Fertilizer Dose (kg ha−1) | Average Yield (kg Grains ha−1) |
Nitrogen Harvested by Yield (kg ha−1) |
Partial Factor Productivity of Nitrogen (PFPN) (kg Grains kg −1) (AN) |
Apparent Use Efficiency of N (APUEN) (%) |
|---|---|---|---|---|
| 50 | 1848 | 50.3 | 37.0 | 100.5 |
| 60 | 1771 | 48.2 | 29.5 | 80.3 |
| 75 | 2112 | 57.4 | 28.2 | 76.6 |
| 80 | 2314 | 62.9 | 28.9 | 78.7 |
| 100 | 2483 | 67.5 | 24.8 | 67.5 |
| 120 | 2749 | 74.8 | 22.9 | 62.3 |
| 150 | 2453 | 66.7 | 16.4 | 44.5 |
| 160 | 2882 | 78.4 | 18.0 | 49.0 |
| 180 | 3413 | 92.8 | 19.0 | 51.6 |
| 200 | 2193 | 59.6 | 11.0 | 29.8 |
| 225 | 2912 | 79.2 | 12.9 | 35.2 |
| 240 | 3300 | 89.8 | 13.8 | 37.4 |
| 300 | 2600 | 70.7 | 8.7 | 23.6 |
| 320 | 3540 | 96.3 | 11.1 | 30.1 |
| 360 | 3783 | 102.9 | 10.5 | 28.6 |
| 400 | 2765 | 75.2 | 6.9 | 18.8 |
| Average | 2695 | 73.3 | 19.7 | 50.9 |
| R2 | 0.83 * | 0.88 ** | 0.83 ** | 0.77 * |
By increasing the nitrogen fertilizer rates, the seed yield increased, reaching an optimum production at 130 kg of N ha−1. However, after this point, the seed yield decreased, as depicted in Figure 2. Similar results were found in trials with a quinoa genotype O3 and two other cultivars [37][42]. The results were adjusted, with a good correlation to the law of diminishing returns (R2 = 0.83, p = 0.00513) [42] and agreement with Pandey et al. [43], who indicated that high rates of nitrogen in crops cause a depressive effect.
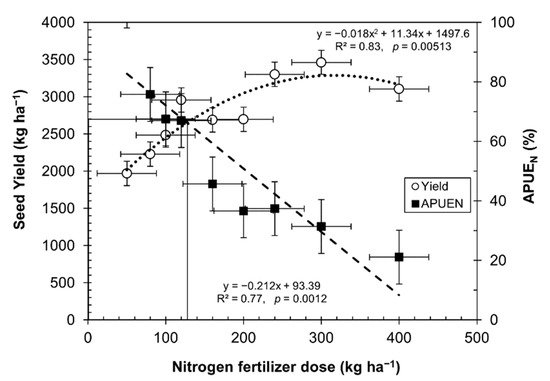
Figure 2. Break-even point between nitrogen fertilizer dose, seed yield, and APUEN (mean ± SE).
Figure 2 depicts the break-even point at which quinoa is efficient enough under certain levels of nitrogen fertilization. Figure 2 shows a higher efficiency in the use of nitrogen in quinoa grown in soils even under very low nitrogen levels. A balance point appeared, indicating that up to 130 kg ha−1 of nitrogen is enough to produce 2700 kg ha−1. This point is a balance between APUEN and seed yield, even when quinoa is grown with high rates of nitrogen fertilizer. There is a remarkable relationship between decreasing APUEN and an increasing amount of nitrogen fertilizer (R2 = 0.77, p = 0.0012).
2.3. Parameters Related to the Uptake and Assimilation of Nitrogen
The PFPN will depend on the physiological efficiency of the cultivar, that is, the proportion of available N absorbed by the crop, and on the losses during the cycle [44]. Nitrogen use efficiency averages 33% in cereals, indicating significant potential for improvement [45]. For quinoa grown on dry land and without an extra contribution of nitrogen fertilization, the PFPN decreases when the nitrogen content in the soil increases (Table 2). With values for PFPN ranging from 59.6 for very nitrogen-poor soil (0.02%) to 6.3 for very nitrogen-rich soil (0.22%), this means a loss of 89.4% in N efficiency.
In a trial with quinoa and five levels of N (0, 40, 80, 120, and 160 kg N ha−1), the highest PFPN was recorded with 40 kg N ha−1 and 30.52 kg of grains produced per kg of N applied [13]. Another study showed that the efficiency of the use of nitrogen in the yield of quinoa with N doses of 0, 50, 100, 150, and 200 kg N ha−1 was affected by higher availability of N in the soil [40]. The data in Figure 2 show a deterioration in PFPN and APUEN when higher doses of fertilizers are included, although the yields increased. For example, applications of 40 kg N ha−1 produced 52 kg of grain for each kilogram of fertilizer applied. This is in contrast to doses of 160 kg N ha−1, where only 17 kg of grain were produced for each kilogram of fertilizer applied, and with 400 kg N ha−1, which produced only 5 kg of grains for each kilogram of nitrogen, that is, a 90.4% efficiency deterioration. The apparent use efficiency of nitrogen (APUEN) shows that the applied or available nitrogen was multiplied 1.4 times in harvested grains. Differently, when 400 kg N ha−1 was applied, only 0.145 times the applied dose was harvested. The 1.4-fold increase in nitrogen content is striking, which could be explained by the presence of microorganisms or the contribution of rain, or by the deepening of the roots to increase the volume of soil to explore.
The data obtained are consistent with those of Franco Alvarado (2018) [39], who applied up to 200 kg N ha−1, finding that the absorption efficiency use of nitrogen (APUEN) decreased as the applied dose of N increased. Without the application of nitrogen fertilizer, it reached the highest APUEN. In contrast, upon application of 200 kg N ha−1, the seed yield decreases. Franco Alvarado [39] found that the optimal dose of available N (62 kg N ha−1) in the soil achieved the highest productivity in quinoa crop. This deterioration in the efficiency indicators indicates that increasing application of nitrogen fertilizers in quinoa is not used to produce grains, it could be derived from the production of biomass [46], or else there is a significant loss of this element by leaching. It has been estimated that between 50% and 70% of the applied nitrogen is lost from the soil–plant system, by surface runoff or leachate or by microbial denitrification, a process by which nitrate is converted to nitrogen oxides (N2O and NO) and elemental nitrogen (N2) is also lost by volatilization [41]. The loss of N by drainage (19.7 g N m−2) represents the main output and the volatilization of urea (8.65 g N m−2) [17].
The efficiency in nitrogen uptake and transfer to grains (APUEN) explains the total nitrogen harvested in the grain compared to the total nitrogen uptake per ton of grain. Table 2 shows that plants with nitrogen deficiency stress have a higher APUEN. The quinoa plants used the little available nitrogen better to produce grains, with a lower yield. The nitrogen-deficient plants showed a decrease in aerial and root biomass and a lower seed yield, but a greater efficiency in the use of nitrogen. Similarly, Calvache and Valle [46] found that as nitrogen increases, the aboveground biomass also increases (Table 3).
Table 3. Effect of nitrogen fertilizer dose application on the production of aboveground dry matter (kg ha−1) in three quinoa varieties grown under irrigated conditions in Ecuador.
| DAS | 20 | 40 | 60 | 80 | 100 | 120 |
|---|---|---|---|---|---|---|
| Nitrogen Fertilizer Dose (kg ha−1) | ||||||
| 0 | 166.6 | 712.7 | 1407.3 | 1835.0 | 3967.5 | 4524.8 |
| 75 | 183.4 | 948.9 | 2226.8 | 3650.8 | 7065.4 | 7832.9 |
| 150 | 221.7 | 1055.7 | 2659.7 | 5002.4 | 9943.7 | 11,366.1 |
Our data resemble those of Alvar-Bertran et al. [41], who compared height and canopy in plants with seed yield. The highest seed yield was concentrated in plants of 40–60 cm with a 3–5% canopy. Calvache and Valle [46] compared the biomass produced by quinoa and seed yield as a function of the nitrogen dose under irrigated or rainfed condition (Figure 3). Unfortunately, the data only reached doses of 150 kg ha−1, which did not allow one to establish, in higher doses, what the real behavior would be. Figure 3 shows that as the dose of nitrogen increased, the production of biomass also increased, while under rainfed and irrigation conditions, the rate of biomass production decreased.
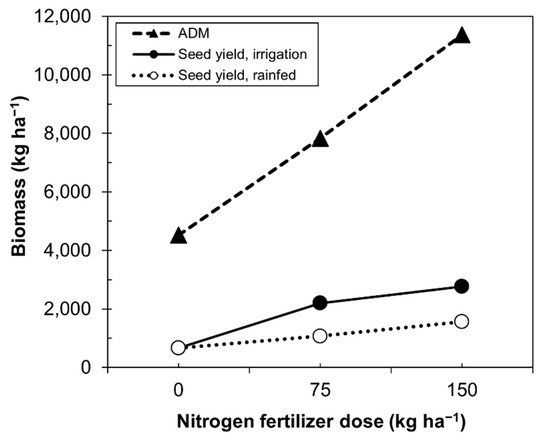
Figure 3. Relationship between the fertilizer dose and quinoa seed yield under rainfed and irrigation conditions. ADM, aboveground dry matter.
Higher doses of nitrogen were derived by the quinoa plants to increase the above vegetative growth rather than to grain production (Figure 3), while decreasing the efficiency of nitrogen for grain production.
The accumulation and redistribution of N are important for the yield and quality of grain [47]. The supply of quinoa grain, like all seed-producing plants, depends on the N accumulated before anthesis. In wheat, approximately 50–95% of the N in grain at harvest comes from the remobilization of the N stored in the shoots and roots before anthesis [47][48]. In quinoa, these values have not been determined; however, data from Calvache and Valle [46] are depicted in Figure 4, where the nitrogen content in the panicle begins to increase from 80 days after sowing, while it remains the same in the stems and decreases in the leaves.
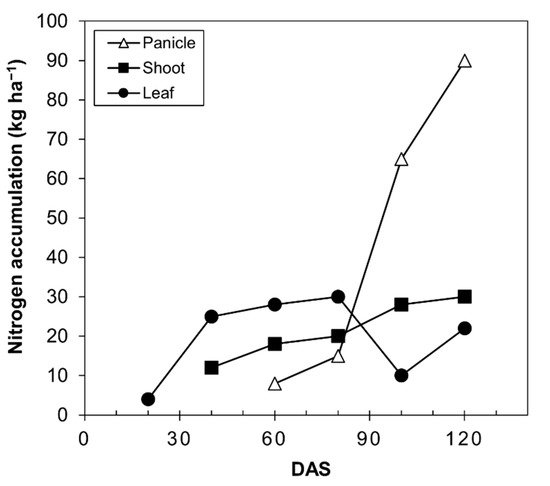
Figure 4. Nitrogen accumulation curve in quinoa plants (Chenopodium quinoa Willd) of the Imbaya variety grown under irrigated conditions. Source: Calvache and Valle [46]. DAS, days after sowing.
In all vegetable species, plant stress accelerates senescence and alters the source–sink relationship, resulting in a significant reduction in crop yield [49]. The southern Bolivian Altiplano has in average of 237 mm of annual rainfall (SENAMHI, Bolivia [50], (www.senamhi.gob.bo, accessed on 15 January 2021), and this is not enough for the cultivation of quinoa. Plants grow under conditions of water deficit, which, added to other environmental factors, creates a condition that accelerates plant senescence because of stress. Naturally, senescence induces the cessation of vegetative growth, accelerates the flowering and fruiting process, changes the plant metabolism, and alters the redistribution and partition of nutrients [51]. Stress senescence affects agronomic characteristics, including the efficiency and yield of carbohydrate/nitrogen use (C/N) and the C/N balance in the source–sink relationship [52][53]. The nitrogen remobilization efficiency (NRE; corresponds to the proportion of N in the crop) depends on the amount of N remobilized to the grain in the period after anthesis and the amount of N stored in the vegetative parts during anthesis. It is important to guarantee that stress senescence has not started prematurely, as nitrogen transportation into the grain will be affected [54]. After the plant takes up nitrogen and metabolizes it into plant proteins, this nitrogen is remobilized to the developing grain [55][56][57].
The growth of fruits and seeds indicates a new sink that competes with the rest of the plant for nutrients. At this point, the nitrogen partition process is important. Bascuñán-Godoy et al. [58] found that the total protein content in quinoa decreases with stress and increases when irrigated again. This decrease correlates with increases in NO3− and NH4+. The increase in NO3− could be associated with a marked stress-induced decrease in nitrate reductase (NR) activity, and the increase in NH4+ is probably associated more with the improvement in the protein degradation and re-assimilation processes of N [59]. Although, it can also be associated with the availability of water, which allows mobilization in the soil to the rhizosphere, improving nitrogen absorption and the presence of microorganisms that provide nitrogen to the plant.
2.4. The Effect of Monoculture on Yielding in Non-Fertilizer Rainfed Cultivation in Bolivian Altiplano
Our study demonstrated how a low soil nitrogen content, as in the southern Bolivian Altiplano, is associated with similar studies [41]. Of the Intersalar soils in the southern Bolivian Altiplano, 91% are sandy loam and sand [26]. The soil texture affects the availability of N by inducing the mineralization and the depth and distribution of the rooting system. Therefore, the application of 120 kg N ha−1 in plots with different soil textures results in differences in nitrogen absorption in quinoa: 134 kg ha−1 for sandy clay loam, 102 kg ha−1 for sandy loam, and 77 kg ha−1 for sand under full irrigation [20]. This situation is important, since the N from deep soil can be absorbed by diffusion and is an important part of the total absorption [60]. Based on applications of 25, 50, and 100 kg N ha−1 in a quinoa crop in Burkina Faso, Alvar-Beltran et al. [41] determined that the nitrogen concentration decreases from 0.051% to 0.037%, in depths from 20 to 60 cm, respectively, for applications of 25 kg ha−1, while for 100 kg ha−1, it decreases from 0.035% to 0.029%.
Under adequate water conditions, the quinoa seed yield increased with higher doses of N, as well as the harvest of N per hectare. When analyzing how the nitrogen deficit affects the conditions of the southern Bolivian highlands, from Table 1, it can be seen that very low values of soil total N (0.02%) equivalent to 14.4 kg N available ha−1 produce average yields of 670 kg of grain ha−1 with an APUEN of 122%, which is surprising, since it contained 22.8% more nitrogen than that provided by the soil. The maximum yield point was obtained with 0.13% of total N in soil, equivalent to 96.5 kg of nitrogen ha−1; with this amount, 1866 kg grain ha−1 was produced, but the APUEN decreased to 52.6. However, these values are close to the equilibrium point shown in Figure 2 for fertilized and irrigated crops (130 kg N ha−1). These values agree with those of Cassman et al. [55], who showed that a low content of N in the soil contributes to an increase in the efficiency of N.
2.5. Sources and Strategies to Improve N Supply and Efficiency in Quinoa in Non-Fertilized Soil in the Altiplano
2.5.1. Sources
Another source of soil N content comes from the atmosphere, which contains 79% by volume of nitrogen, making it a source of great reserve for the system, since it feeds the nitrogen cycle. In the Bolivian Altiplano, there is no history or records about the contribution of nitrogen by rainwater and the atmosphere [61]. The rainfall in the 2016 and 2017 seasons was between 194 and 280 mm year−1 (SENAMHI, Bolivia, www.senamhi.gob.bo, accessed on 15 January 2021). This low level of rainfall makes it difficult to evaluate the contribution of N. There are many controversies about the amount of N deposited through this way in soil. In temperate climates, it can fluctuate between 0.74 and 21 kg N ha−1 year−1 [62], and 15 kg N ha−1 year−1 [63] could be considered the average. These amounts would be higher in tropical climates, i.e., between 6.5 and 72 kg N ha−1 year−1 [62].
In the Bolivian Altiplano, electric shocks can be intense [64], but the amount of rain is much less. It is unknown whether the factor of electrical discharge and/or static electricity results in a higher nitrogen contribution, or why a scarcity of nitrogen is observed in Altiplano soils, which is significant in the soil nitrogen balance [61]. The electrical discharge that occurs during storms synthesizes nitrogen oxides from nitrogen (N2) and oxygen (O2) in the air, being driven into the ground by rain [65][66]. The quantity of nitrate produced across the world is estimated to 7.5 million ton per year.
2.5.2. Strategies
Interestingly, other parameters such as root biomass only correlate with the seed yield under low nitrate conditions, but not at sufficient levels of nitrate [67]. It has been published that root biomass is not important for the uptake of N [68], or even that plants cannot uptake N during grain filling [69]. However, Mi et al. [70] reported that root biomass is an important attribute for N uptake in corn at low nitrate levels (but not at sufficient nitrate levels). The roots of maize can take up N even during the reproductive phase [10]. Coke and Gallais [71] estimated that 62% of the N in the kernel originates from N remobilization, and 38% is derived from post-silking root N uptake. Recently, it has been reported that the increase in the number of secondary roots is related to the upregulation of nitrate transporter gene (CqNRT2) under low nitrate conditions in quinoa seedlings of both Socaire (an Andean landrace) and Faro [72]. This indicates that a low amount of N induces in quinoa a series of mechanisms to cope with low N.
There are antecedents that relate nitrogen deficiency with other active compounds such as Strigolactones (SL). These hormones act by activating the signaling pathways that allow lipid catabolism to be the main carbon source in fungi. Under nutrient deprivation conditions, the production of large amounts of SL leads to the suppression of shoot branching and stimulates symbiosis [73][74]. Strigolactones promote the modification of the architecture of roots and shoots and stimulate a symbiosis of rhizobia bacteria and AMF fungi, and SLs play a crucial role in nitrogen and phosphorus deficiency.
Another of the strategies used by halophytes to capture nutrients is the association with soil microorganisms, especially arbuscular mycorrhizal fungi (AMF), which promotes growth and development under stressful conditions [75][76][77], and plant growth-promoting rhizobacteria (PGPR), with the ability to colonize the roots of many plant species, contributing to their development and survival [41].
The participation of arbuscular mycorrhizal fungi (AMF) in quinoa, a facultative halophyte, is debatable, since the presence of root symbiont fungi in Bolivian Andean quinoa plants is insignificant [78], and plant growth responses could be considered a mutualism–parasitism continuum [79]. However, some research, e.g., in the desert zone of Chile, has determined that there is a high presence of mycotrophic plant species with a high variation in the degree of mycorrhization in the root (mycorrhizal colonization and the mycorrhizal medium), through the production of resistance spores and extraradical mycelium [80]. Despite the low level of AMF colonization, it has been proposed that quinoa could be an interesting component for crops rotation to improve and increase N cycling in soils compared to other crops [81].
In quinoa, in particular, there are very few investigations on the presence of fungi and their contribution to growth or to withstand stressful conditions. The dominant fungal genera (Penicillium, Phoma, and Fusarium) have been detected in the roots of quinoa [82]; for example, Macia-Vicente et al. [83] and Khan et al. [84] previously found them as root inhabitants in several plant species. These fungal genera play a positive role in plant growth and tolerance to abiotic stress. The endophyte fungus community has been recognized as one of the Chilean quinoa ecotypes [82]. Despite a relatively high diversity of endophytic root fungi associated with quinoa plants, the dominant fungal community consists of only Ascomycotaphyla. The most abundant fungal genera in quinoa are Penicillium, Phoma, and Fusarium, which are common endophytes in plant roots, highlighting endophytic root fungi as a new additional performer [83].
Furthermore, there is a history of the participation of bacterial endophytes associated with quinoa [83][84]; 100% of quinoa seeds are inhabited by several bacteria from the genus Bacillus [83], which probably induces a state of natural readiness in quinoa plants, allowing them to overcome extreme environmental situations. Among the best-known microorganisms with PGPR activity are species of the genera Rhizobium sp., Azospirillum sp., and Pseudomonas sp. [85][86]. There are several mechanisms by which bacteria contribute to the germination, growth, and survival of plants, including biological nitrogen fixation, solubilization of phosphates, production of siderophores, biosynthesis of phytohormones (auxins, cytokines, and gibberellins), synthesis of antibiotics, and induction of systemic resistance [87][88]. Under low nitrogen concentrations, auxin biosynthesis and transcriptional accumulation are induced, thus regulating lateral root formation. Conversely, lateral root growth can be inhibited with a higher than optimal supply of N [89][90]. Nitrate transporter (NRT) genes have also been reported to be responsible for the high affinity of the NO3− transport system, which is related to the growth of lateral roots [72][91][92]. Based on the information provided, it is possible to assume that part of the nitrogen supply of quinoa in conditions of deficit of this element, is supplied by the interaction that occurs with these microorganisms.
This entry is adapted from the peer-reviewed paper 10.3390/plants10112479
References
- Gutiérrez Castorena, E.V.; Gutiérrez Castorena, M.d.C.; Ortiz Solorio, C.A. Manejo integrado de nutrientes en sistemas agrícolas intensivos: Revisión. Rev. Mex. Cienc. Agrícol. 2015, 6, 201–215.
- Kraiser, T.; Gras, D.E.; Gutierrez, A.G.; Gonzalez, B.; Gutierrez, R.A. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 2011, 62, 1455–1466.
- Robertson, G.P.; Groffman, P.M. Chapter 14: Nitrogen Transformations. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Burlington, MA, USA, 2015; pp. 421–446.
- Delon, C.; Galy-Lacaux, C.; Boone, A.; Liousse, C.; Serça, D.; Adon, M.; Diop, B.; Akpo, A.; Lavenu, F.; Mougin, E.; et al. Atmospheric nitrogen budget in Sahelian dry savannas. Atmos. Chem. Phys. 2010, 10, 2691–2708.
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 2004, 141, 221–235.
- Jaeglé, L.; Martin, R.V.; Chance, K.; Steinberger, L.; Kurosu, T.P.; Jacob, D.J.; Modi, A.I.; Yoboué, V.; Sigha-Nkamdjou, L.; Galy-Lacaux, C. Satellite mapping of rain-induced nitric oxide emissions from soils. J. Geophys. Res. Atmos. 2004, 109, 1–10.
- Galy-Lacaux, C.; Delon, C.; Solmon, F.; Adon, M.; Yoboué, V.; Mphepya, J.; Pienaar, J.; Diop, B.; Sigha, L.; Dungall, L.; et al. Dry and Wet Atmospheric Nitrogen Deposition in West Central Africa. In Proceedings of the 6th International Nitrogen Conference, Kampala, Uganda, 18 November 2013.
- Karunarathne, S.D.; Han, Y.; Zhang, X.-Q.; Li, C. Advances in Understanding the Molecular Mechanisms and Potential Genetic Improvement for Nitrogen Use Efficiency in Barley. Agronomy 2020, 10, 662.
- Cabrera, M.L. Mineralización y nitrificación: Procesos claves en el ciclo del nitrógeno. In Proceedings of the Simposio Fertilidad 2007. IPNI Cono Sur-Fertilizar AC. Seminario Internacional de Nutricion Vegetal 2007. Rosario, Acassuso, Argentina, 10–11 May 2007; pp. 1–9.
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157.
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108.
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 97–185.
- Grahmann, K.; Verhulst, N.; Buerkert, A.; Ortiz-Monasterios, I.; Govaerts, B. Nitrogen use efficiency and optimization of nitrogen fertilization in conservation agriculture. CAB Rev. 2013, 53, 6.
- Eshel, A.; Beeckman, T. Plant Roots: The Hidden Half, 4th ed.; CRC Press, Taylor and Francis Group: Abingdon, UK, 2013; p. 848.
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Secur. 2020, 26, 100429.
- FAO/ORALC. La Quinua: Cultivo Milenario para Contribuir a la Seguridad Alimentaria Mundial; Oficina Regional para América Latina y el Caribe: Santiago, Chile, 2011.
- Rodriguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and Resilient Cereals and Pseudo-Cereals for Marginal Agriculture: Molecular Advances for Improving Nutrient Bioavailability. Front. Genet. 2020, 11, 49.
- Geerts, S.; Raes, D.; Garcia, M.; Mendoza, J.; Huanca, R. Crop water use indicators to quantify the flexible phenology of quinoa (Chenopodium quinoa Willd.) in response to drought stress. Field Crop. Res. 2008, 108, 150–156.
- Jacobsen, S.E. Adaptación y Posibilidades para la Quinua en las Latitudes Septentrionales de Europa. In Estado del Arte de la Quinua en el Mundo en 2013; Bazile, D., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 520–533.
- Razzaghi, F.; Plauborg, F.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effect of nitrogen and water availability of three soil types on yield, radiation use efficiency and evapotranspiration in field-grown quinoa. Agric. Water Manag. 2012, 109, 20–29.
- Rodriguez, J.P.; Ono, E.; Abdullah, A.M.S.; Choukr-Allah, R.; Abdelaziz, H. Cultivation of Quinoa (Chenopodium quinoa) in Desert Ecoregion. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Environment & Policy; Springer International Publishing: Cham, Switzerland, 2020; Volume 58, pp. 145–161.
- Geerts, S.; Raes, D.; Garcia, M.; Del Castillo, C.; Buytaert, W. Agro-climatic suitability mapping for crop production in the Bolivian Altiplano: A case study for quinoa. Agric. For. Meteorol. 2006, 139, 399–412.
- Andressen, R.; Monasterio, M.; Terceros, L.F. Regímenes climáticos del altiplano sur de Bolivia: Una región afectada por la desertificación. Rev. Geogr. Venez. 2007, 48, 11–32.
- Winkel, T.; Alvarez-Flores, R.; Bommel, P.; Bourliaud, J.; Chevarria Lazo, M.; Cortes, G.; Cruz, P.; Del Castillo, C.; Gasselin, P.; Joffre, R.; et al. Altiplano Sur de Bolivia. In Estado del Arte de la Quinua en el Mundo en 2013’; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO: Montpellier, France; CIRAD: Santiago, Chile, 2014; pp. 432–449.
- Kerssen, T.M. Food sovereignty and the quinoa boom: Challenges to sustainable re-peasantisation in the southern Altiplano of Bolivia. Third World Q. 2015, 36, 489–507.
- Cárdenas, J.; Choque, W.; Guzmán, R. Fertilidad, Uso y Manejo de Suelos en la Zona del Intersalar, Departamentos de: Oruro y Potosí; Fundación FAUTAPO: La Paz, Bolivia, 2008; p. 113.
- Ministerio de Desarrollo Rural y Tierras (MDRyT); Consejo Nacional de Comercializadores y Productores de Quinua (CONACOPROQ). Política Nacional de la Quinua; Ministerio de Desarrollo Rural y Tierras (MDRyT): La Paz, Bolivia; Consejo Nacional de Comercializadores y Productores de Quinua (CONACOPROQ): La Paz, Bolivia, 2009; p. 134.
- Echalar, M.; Torrico, J.; Martínez, F. Analisis de la sostenibilidad de la produccion de quinua (Chenopodium quinoa) en el intersalar Boliviano. Cienciagro 2011, 2, 303–312.
- Miranda, R.; Carlesso, R.; Huanca, M.; Mamani, P.; Borda, A. Yield and nitrogen accumulation in quinoa (Chenopodium quinoa Willd.) produced with manure and supplementary irrigation. Venesuelos 2012, 20, 21–29.
- Del Barco-Gamarra, M.T.; Foladori, G.; Soto-Esquivel, R. Insustentabilidad de la producción de quinua en Bolivia. Estud. Sociales. Rev. Aliment. Contemp. Desarro. Reg. 2019, 29, 2–26.
- Alejo Inda, R. Evaluación del Comportamiento del Nitrógeno, en Parcelas con Cultivo de Quinua bajo Diferente Manejo de Suelos (Municipios Salinas de Garci Mendoza), Oruro. Engineering Thesis, Universidad Mayor de San Andrés, Facultad de Agronomía, La Paz, Bolivia, 2010.
- Borda López, F.M. Niveles de Urea y Guano de Isla con y sin Zeolita en el Rendimiento de Quinua (Chenopodium quinoa Willd) Canaria 3200 msnm-Ayacucho. Engineering Thesis, Universidad Nacional de San Cristobal de Huamanga, Ayacucho, Peru, 2018.
- Campillo, R.; Contreras, G. Capitulo 4: Gestión nitrogenada y potásica del cultivo de quinoa en La Araucanía. In Quinoa del Sur de Chile: Alternative Productiva y Agroindustrial de Alto Valor; Diaz, S.J., Ed.; Colección Libros INIA-Instituto de Investigaciones Agropecuarias. Centro Regional de Investigacion Carillanca: Temuco, Chile, 2019; pp. 45–68.
- Jacobsen, S.E.; Jørgensen, I.; Stølen, O. Cultivation of quinoa (Chenopodium quinoa) under temperate climatic conditions in Denmark. J. Agric. Sci. 2009, 122, 47–52.
- Auf’m Erley, G.S.; Kaul, H.-P.; Kruse, M.; Aufhammer, W. Yield and nitrogen utilization efficiency of the pseudocereals amaranth, quinoa, and buckwheat under differing nitrogen fertilization. Eur. J. Agron. 2005, 22, 95–100.
- Berti, M.; Wilckens, R.; Hevia, F.; Serri, H.; Vidal, I.; Méndez, C. Nitrogen fertilization in quinoa (Chenopodium quinoa Willd). Ciencia e Investigación Agraria 2000, 27, 81–90.
- Mendoza Nieto, E.; Luis Olivas, D.; Mejía Domínguez, C.M.; García Cochagne, J. Fertilización nitrogenada en el rendimiento de dos variedades de quinua. Infinitum 2016, 6, 11–15.
- Llaca Ninaja, G.C. Influencia de la Fertilización Nitrogenada y Fosfórica en el Rendimiento de Quinua (Chenopodium quinoa Willd) en el Proter Sama, Región Tacna. Engineering Thesis, Universidad Nacional Jorge Basadre Grohmann-Tacna, Tacna, Peru, 2014.
- Franco Alvarado, L.A. Eficiencia de Utilizacion del Nitrogeno en el Rendimiento de Quinua (Chenopodium quinoa Willd) Adaptada a la Zona Norte de la Provincia de Los Rios. Engineering Thesis, Universidad Tecnica Estatal de Quevedo, Quevedo, Los Rios, Ecuador, 2018.
- Herreros Quispe, A.L. Fertilización Nitrogenada y Fosfórica en Quinua (Chenopodium quinoa willd.) CV. Salcedo INIA” bajo Riego a Goteo en Zona Árida. Engineering Thesis, Universidad Nacional de San Agustin de Arequipa, Arequipa, Peru, 2018.
- Alvar-Beltrán, J.; Saturnin, C.; Dao, A.; Dalla Marta, A.; Sanou, J.; Orlandini, S. Effect of drought and nitrogen fertilisation on quinoa (Chenopodium quinoa Willd.) under field conditions in Burkina Faso. Ital. J. Agrometeorol. 2019, 1, 33–43.
- Oyarzun Arrechea, M. Respuesta Productiva de un Cultivo de Maíz (“Zea mays” L. Var. Dracma) a Distintas Dosis de Nitrógeno con dos Tipos de Riego (Aspersión e Inundación) y Efecto sobre la Lixiviación de Nitratos. Engineering Thesis, Universidad Publica de Navarra, Navarra, Spain, 2010.
- Pandey, R.K.; Maranville, J.W.; Bako, Y. Nitrogen fertilizer response and use efficiency for three cereal crops in Niger. Commun. Soil Sci. Plant Anal. 2001, 32, 1465–1482.
- Food and Agriculture Organization of the United Nations (FAO). El Manejo del Suelo en la Producción de Hortalizas con Buenas Prácticas Agrícolas; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013.
- Quintero, C.; Boschetti, G. Eficiencia de uso del Nitrógeno en Trigo y Maíz en la Región Pampeana Argentina. Facultad de Ciencias Agropecuarias UNER. 2009. Available online: https://www.researchgate.net/profile/Cesar-Quintero-4/publication/266357692_Eficiencia_de_uso_del_Nitrogeno_en_Trigo_y_Maiz_en_la_Region_Pampeana_Argentina/links/58e3c7c0458515b725b038bf/Eficiencia-de-uso-del-Nitrogeno-en-Trigo-y-Maiz-en-la-Region-Pampeana-Argentina.pdf (accessed on 7 January 2021).
- Calvache Ulloa, M.; Valle, L. Índice de cosecha con macro-nutrientes en grano de quinua (Chenopodium quinoa Willd). Rev. Alfa 2021, 5, 15–28.
- Critchley, C.S. A Physiological Explanation for the Canopy Nitrogen Requirement of Winter Wheat. Ph.D. Thesis, University of Nothingham, Nottingham, UK, 2001. Available online: http://eprints.nottingham.ac.uk/12834/1/368341.pdf (accessed on 21 January 2021).
- Kichey, T.; Hirel, B.; Heumez, E.; Dubois, F.; Le Gouis, J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crop. Res. 2007, 102, 22–32.
- Sade, N.; Del Mar, M.R.-W.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853.
- SENAMHI. Yearly Data on Rainfall, Oruro. 2017. Available online: http://senamhi.gob.bo/index.php/boletines (accessed on 15 January 2021).
- Guiboileau, A.; Sormani, R.; Meyer, C.; Masclaux-Daubresse, C. Senescence and death of plant organs: Nutrient recycling and developmental regulation. Comptes Rendus Biol. 2010, 333, 382–391.
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337.
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622.
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crop. Res. 2014, 155, 213–223.
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140.
- Stewart, W. Consideraciones en el uso eficiente de nutrientes. Inf. Agronóm. 2007, 67, 1–10.
- Puentes-Páramo, Y.; Menjivar-Flores, J.; Aranzazu-Hernández, F. Eficiencias en el uso de nitrógeno, fósforo y potasio en clones de cacao (Theobroma cacao L.). Bioagro 2014, 26, 99–106.
- Bascunan-Godoy, L.; Reguera, M.; Abdel-Tawab, Y.M.; Blumwald, E. Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa Willd. Planta 2016, 243, 591–603.
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013, 163, 1609–1622.
- Kristensen, H.L.; Thorup-Kristensen, K. Root Growth and Nitrate Uptake of Three Different Catch Crops in Deep Soil Layers. Soil Sci. Soc. Am. J. 2004, 68, 529–537.
- Hervé, D.; Mita, V.; Coûteaux, M.-M. Construcción de un balance de nitrógeno en cultivos de papa bajo rotación con largo descanso. Ecol. Boliv. 2006, 41, 133–153.
- Fassbender, H.W. Química de Suelos, con Énfasis en Suelos de América Latina; IICA: San Jose, Costa Rica, 1986; p. 398.
- Wolf, J.; Van Keulen, H. Modeling long-term crop response to fertilizer and soil nitrogen. Plant Soil 1989, 120, 23–38.
- VAISALA. Lightning like Never Before. Annual Lightning Report 2020; VAISALA: Vaanta, Finland, 2021; p. 25.
- Hill, R.D.; Rinker, R.G.; Wilson, H.D. Atmospheric Nitrogen Fixation by Lightning. J. Atmos. Sci. 1980, 37, 179–192.
- Drapcho, D.L.; Sisterson, D.; Kumar, R. Nitrogen fixation by lightning activity in a thunderstorm. Atmos. Environ. 1983, 17, 729–734.
- Bascunan-Godoy, L.; Sanhueza, C.; Pinto, K.; Cifuentes, L.; Reguera, M.; Briones, V.; Zurita-Silva, A.; Alvarez, R.; Morales, A.; Silva, H. Nitrogen physiology of contrasting genotypes of Chenopodium quinoa Willd. (Amaranthaceae). Sci. Rep. 2018, 8, 17524.
- Borrell, A.; Hammer, G.; Oosterom, E. Stay-green: A consequence of the balance between supply and demand for nitrogen during grain filling? Ann. Appl. Biol. 2001, 138, 91–95.
- Triboi, E.; Martre, P.; Girousse, C.; Ravel, C.; Triboi-Blondel, A.-M. Unravelling environmental and genetic relationships between grain yield and nitrogen concentration for wheat. Eur. J. Agron. 2006, 25, 108–118.
- Mi, G.H.; Chen, F.J.; Liu, J.A.; Tong, Y.P. Biological potential of nitrogen utilization in crops and its genetic improvement. In Explore Biological Potential for Soil Nutrient Utilization and Maintain Nutrient Recycling in Soil Environment; Li, Z., Ed.; China Agricultural Press: Beijing, China, 2004; pp. 202–216.
- Coque, M.; Gallais, A. Genetic Variation for Nitrogen Remobilization and Postsilking Nitrogen Uptake in Maize Recombinant Inbred Lines: Heritabilities and Correlations among Traits. Crop Sci. 2007, 47, 1787–1796.
- Pinto-Irish, K.; Coba de la Pena, T.; Ostria-Gallardo, E.; Ibanez, C.; Briones, V.; Vergara, A.; Alvarez, R.; Castro, C.; Sanhueza, C.; Castro, P.A.; et al. Seed characterization and early nitrogen metabolism performance of seedlings from Altiplano and coastal ecotypes of Quinoa. BMC Plant Biol. 2020, 20, 343.
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200.
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194.
- Hildebrandt, U.; Janetta, K.; Ouziad, F.; Renne, B.; Nawrath, K.; Bothe, H. Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 2001, 10, 175–183.
- Plenchette, C.; Duponnois, R. Growth response of the saltbush Atriplex nummularia L. to inoculation with the arbuscular mycorrhizal fungus Glomus intraradices. J. Arid. Environ. 2005, 61, 535–540.
- Gill, S.; Alshankiti, A.; Shahid, S.A.; Rodriguez, J.P. Amending Soil Health to Improve Productivity of Alternate Crops in Marginal Sandy Soils of the UAE. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Environment & Policy; Springer International Publishing: Cham, Switzerland, 2020; pp. 93–123.
- Urcelay, C.; Acho, J.; Joffre, R. Fungal root symbionts and their relationship with fine root proportion in native plants from the Bolivian Andean highlands above 3,700 m elevation. Mycorrhiza 2011, 21, 323–330.
- Kellogg, J.A.; Reganold, J.P.; Murphy, K.M.; Carpenter-Boggs, L.A. A Plant-Fungus Bioassay Supports the Classification of Quinoa (Chenopodium quinoa Willd.) as Inconsistently Mycorrhizal. Microb. Ecol. 2021, 82, 135–144.
- Santander, C.; Olave, J.; García, S.; Vidal, C.; Aguilera, P.; Borie, F.; Cornejo, P. Micorrizas arbusculares y su efecto nodriza en condiciones hídricas limitantes. Exp. Rev. Transf. Cient. 2014, 4, 59–61.
- Wieme, R.A.; Reganold, J.P.; Crowder, D.W.; Murphy, K.M.; Carpenter-Boggs, L.A. Productivity and soil quality of organic forage, quinoa, and grain cropping systems in the dryland Pacific Northwest, USA. Agric. Ecosyst. Environ. 2020, 293, 106838.
- Gonzalez-Teuber, M.; Vilo, C.; Bascunan-Godoy, L. Molecular characterization of endophytic fungi associated with the roots of Chenopodium quinoa inhabiting the Atacama Desert, Chile. Genom. Data 2017, 11, 109–112.
- Pitzschke, A. Developmental Peculiarities and Seed-Borne Endophytes in Quinoa: Omnipresent, Robust Bacilli Contribute to Plant Fitness. Front. Microbiol. 2016, 7, 2.
- Ortuño, N.; Claros, M.; Gutiérrez, C.; Angulo, M.; Castillo, J. Bacteria associated with the cultivation of quinoa in the Bolivian Altiplano and their biotechnological potential. Rev. Agric. 2014, 53, 53–61.
- Bashan, Y.; De-Bashan, L. Plant growth-promoting. Encycl. Soils Environ. 2005, 1, 103–115.
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117.
- GarciA, G.N.; Navarro García, S. Química Agrícola: Química del Suelo y de los Nutrientes Esenciales para las Plantas; Mundi-Prensa Libros: Madrid, Spain, 2013.
- Ribaudo, C.M.; Riva, D.S.; Curá, J.A.; Ponds, C.; Granell-Richard, A.; Cantora, M. Etileno como mediador de los mecanismos directos e indirectos de la promoción del crecimiento vegetal ejercido por rizobacterias. In Rizósfera, Biodiversidad y Agricultura Sustentable; García de Salamone, I.E., Vázquez, S., Penna, C., Cassán, F., Eds.; Asociación Argentina de Microbiología Pág: Buenos Aires, Argentina, 2013; pp. 215–240.
- Guo, Y.; Chen, F.; Zhang, F.; Mi, G. Auxin transport from shoot to root is involved in the response of lateral root growth to localized supply of nitrate in maize. Plant Sci. 2005, 169, 894–900.
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wiren, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179.
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutierrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482.
- Hu, S.; Zhang, M.; Yang, Y.; Xuan, W.; Zou, Z.; Arkorful, E.; Chen, Y.; Ma, Q.; Jeyaraj, A.; Chen, X.; et al. A novel insight into nitrogen and auxin signaling in lateral root formation in tea plant . BMC Plant Biol. 2020, 20, 232.
This entry is offline, you can click here to edit this entry!
