Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Reproductive Biology
Endometriosis is a common disorder defined as the presence of endometrial tissue (glandular cells and stroma) outside the uterine cavity.
- caffeine
- coffee
- caffeine-containing beverages
- endometriosis
- environmental factors
1. Introduction
Endometriosis is a common disorder defined as the presence of endometrial tissue (glandular cells and stroma) outside the uterine cavity [1]. The most common sites of endometriosis include the pelvic peritoneum, the ovaries, and the uterosacral ligaments. Women with endometriosis may be asymptomatic or suffer from subfertility, pelvic pain, and dyspareunia [2]. The disease affects 2–10% of women of reproductive age and 30–50% of the female population in general, but the actual prevalence is unknown, because the diagnosis is only established by surgery [3].
While the definitive cause of endometriosis constitutes a matter of debate, its clinical heterogeneity suggests a multifactorial causal background that consists of both genetic and environmental factors [4]. During the last two decades, several studies have correlated endometriosis with modifiable risk factors, such as food intake and lifestyle habits, given their potential influence on hormonal levels, immune response, and inflammatory activity [5][6][7].
Caffeine, one of the most widely used pharmacologically active substances worldwide, has been studied extensively as a potential contributing factor linked with the development of hormone-dependent conditions [8]. This theory stems from the fact that caffeine affects the levels of steroid hormones, the production of the sex hormone-binding globulin in the liver, and the conversion of androgens to estrogens by altering aromatase function [9][10].
2. Caffeine and Endometriosis Analysis
In the primary analysis, overall caffeine intake (>100 mg/day) increased the risk of endometriosis by 12%, but this difference was not statistically significant (10 studies; 38,601 participants; RR 1.12, 95% CI 0.97–1.28; I2 = 70%) (Figure 1) when compared to little or no caffeine use (<100 mg/day). Substantial heterogeneity was observed.
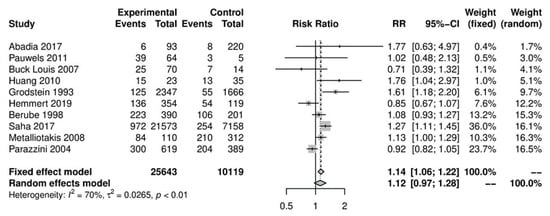
Figure 1. Main analysis of events (endometriosis cases) in caffeine intake group (>100 mg/day) versus little or no caffeine intake (<100 mg/day).
We performed further subgroup analyses to stratify according to the level of caffeine intake (Figure 2). High caffeine consumption (>300 mg/day) significantly increased the risk of endometriosis when compared to little or no caffeine (<100 mg/day) (five studies; 15,085 participants; RR 1.30, 95% CI 1.04–1.63; I2 = 56%). Moderate caffeine intake (100–300 mg/day) also increased the risk of endometriosis but the difference did not reach significance (five studies, 29,920 participants, RR 1.18, 95%CI 0.99–1.40, I2 = 37%). However, 95% prediction intervals failed to exclude the null value (high caffeine intake 95% PI (0.77–2.22); moderate caffeine intake 95% PI (0.85–1.63)), which was possibly due to the high heterogeneity observed.
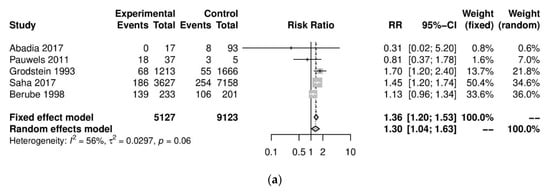
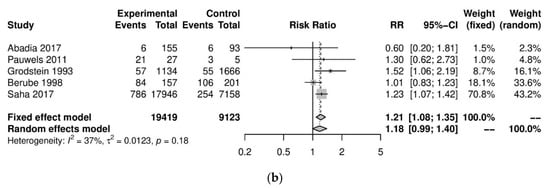
Figure 2. Analysis of (a) high caffeine intake (>300 mg/day) versus little or no caffeine (<100 mg/day) and (b) moderate caffeine intake (200–300 mg/day) versus little or no caffeine (<100 mg/day).
We performed a series of sensitivity analyses for the primary analysis which showed consistent results and did not reduce the high heterogeneity between studies. Sensitivity analyses on the risk of endometriosis in women with high/moderate versus little or no caffeine consumption were conducted based on country (Americas: RR 1.10, 95% CI 0.92–1.31, I2 = %; Europe: RR 1.07, 95% CI 0.8–1.44, I2 = 85%); study design (case-control studies: RR 1.19, 95% CI 0.94–1.5, I2 = 75%; cohort studies: RR 1.07, 95% CI 0.88–1.3, I2 = 67%); type of diagnosis (surgical diagnosis: RR 1.08, 95% CI 0.93–1.25, I2 = 67%); MINORS score of the study (moderate risk of bias: RR 1.04 95% CI 0.82–1.32, I2 = 82%), and coffee as the only caffeine source (RR 1.15 95%, CI 0.95–1.39, I2 = 81%). No evidence of small-study effects was observed in the main analysis (Egger’s p = 0.97).
This entry is adapted from the peer-reviewed paper 10.3390/nu13103457
References
- Dunselman, G.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412.
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398.
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41.
- Asghari, S.; Valizadeh, A.; Aghebati-Maleki, L.; Nouri, M.; Yousefi, M. Endometriosis: Perspective, lights, and shadows of etiology. Biomed. Pharmacother. 2018, 106, 163–174.
- Soave, I.; Occhiali, T.; Wenger, J.-M.; Pluchino, N.; Caserta, D.; Marci, R. Endometriosis and food habits: Can diet make the difference? J. Endometr. Pelvic Pain Disord. 2018, 10, 59–71.
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336.
- Kechagias, K.S.; Semertzidou, A.; Athanasiou, A.; Paraskevaidi, M.; Kyrgiou, M. Bisphenol-A and polycystic ovary syndrome: A review of the literature. Rev. Environ. Health 2020, 35, 323–331.
- Cano-Marquina, A.; Tarín, J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21.
- Ferrini, R.L.; Barrett-Connor, E. Caffeine intake and endogenous sex steroid levels in postmenopausal women The Rancho Bernardo Study. Am. J. Epidemiol. 1996, 144, 642–644.
- Wedick, N.M.; Mantzoros, C.S.; Ding, E.L.; Brennan, A.M.; Rosner, B.; Rimm, E.B.; Hu, F.B.; Van Dam, R.M. The effects of caffeinated and decaffeinated coffee on sex hormone-binding globulin and endogenous sex hormone levels: A randomized controlled trial. Nutr. J. 2012, 11, 86.
This entry is offline, you can click here to edit this entry!
