Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Communication between cancer cells and the surrounding stromal cells of the tumor microenvironment (TME) plays a key role in promoting metastasis, which is the major cause of cancer death. Small membrane-bound particles called extracellular vesicles (EVs) are released from both cancer and stromal cells and have a key role in mediating this communication through transport of cargo such as various RNA species (mRNA, miRNA, lncRNA), proteins, and lipids.
- extracellular vesicles
- cancer
- metastasis
- tumor microenvironment
- cellular communication
1. Introduction
An important prognostic determinant for cancer is the development of secondary metastatic tumors that arise in a separate location from the original primary lesion [1]. Following remission of a primary tumor, metastatic cancer may take years or decades to develop and is extremely difficult to treat. Cancer is the second leading cause of death in the world, and metastasis is responsible for 90% of cancer-related mortality [2]. In particular, lymph node metastasis is correlated with a significant decrease in the survival rate of cancer patients due to the potential for dissemination to multiple distant sites [3].
The process of metastasis, also known as the metastatic cascade, involves multiple steps: a local invasion of tumor cells from the primary tumor to surrounding tissues, intravasation into the circulatory system, arrest of the tumor cell at a site along the endothelium of the blood vessel, and extravasation into the surrounding tissue. At this time, a new secondary tumor can begin to grow within the permissive environment of the pre-metastatic niche (PMN) [5,6]. Key processes involved in the steps of this cascade include epithelial–mesenchymal transition (EMT), which facilitates invasion of tumor cells from the primary tumor into surrounding tissues through a transformation of tumor cells to a migratory phenotype; angiogenesis, in which the formation of new blood vessels supplies nutrients to the primary tumor, as well as allowing intravasation and transport of the cancer cells to a distant site; and formation of the pre-metastatic niche which has supportive conditions for cancer cell growth at the site of future metastatic colonization [4]. These processes are not specific to a particular cell or cellular function but are rather a coordinated series of events requiring collective efforts and reciprocal signaling from the surrounding, dynamic tumor microenvironment (TME).
The TME is the area that encircles the tumor and contains both non-cellular and cellular components that can promote tumor progression and metastasis, with increasing evidence showing their involvement in drug resistance [7]. The extracellular matrix (ECM) makes up the non-cellular component and primarily consists of water, proteins, and polysaccharides, with the exact composition unique to the tissue type. The cellular aspect is composed of endothelial cells of the blood and lymph vessel networks, immune cells, fibroblasts, neuroendocrine cells, adipose cells, and additional factors [8]. Tumor cells and non-tumor components of the TME communicate bi-directionally to facilitate the invasion-metastasis cascade, as well as the formation and maintenance of the tumor microenvironment [7]. Complex networks of direct cell–cell contact and the release of soluble factors make up the different channels of intercellular communication [9]. These molecules are traditionally thought of as inflammatory regulators, growth factors, ECM remodeling enzymes, cytokines, and chemokines. Advances in research methods are revealing new mechanisms of communication that aid in the understanding of tumor biology. For instance, circulating tumor cells and cell-free circulating tumor DNA are shed from the solid tumor into circulation [8,10]. An emerging channel of intercellular communication is the trafficking of extracellular vesicles (EVs).
EVs are small, spherical, membrane-bound particles that are secreted into the extracellular space and deliver biologically active molecules to both nearby and distant sites [11]. EVs encompass both exosomes, which originate from the endosomal system of the cell, and microvesicles, which bud directly off the plasma membrane [12]. Cellular communication via EVs is known to modify the composition of the TME through diverse cargo that includes proteins, DNA fragments, mRNA, small RNAs (including microRNAs [miRNAs]), long noncoding RNAs (lncRNAs), circular RNAs (circRNA), lipids, and metabolites [13,14]. Receptors and ligands on the surface of the EV facilitate specific targeting for biodistribution and can trigger signaling changes within the recipient cell. Alternatively, vesicle contents are released within the recipient cell after endocytic uptake of the EVs [13]. The mode of communication can be either paracrine, targeting cells of different types in the same region, or endocrine, targeting different cell types in different anatomical locations [15]. Thus, EVs play an essential role in both primary tumor growth and metastatic evolution (Figure 1). As a result, targeting the reciprocal communication between tumor cells and TME is a promising field for the development of new therapeutics [9].
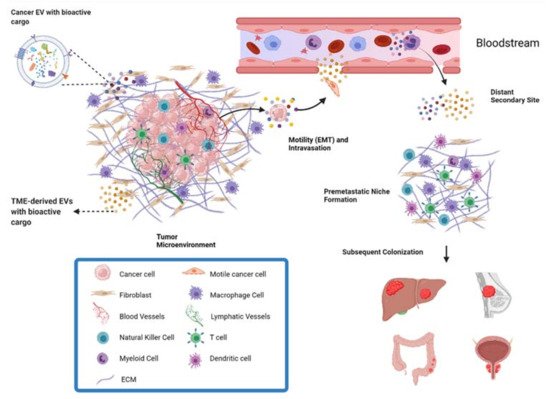
Figure 1. Overview of metastasis and contribution of EVs. EVs derived from both cancer cells and the cells of the TME can facilitate metastasis. EVs can act on surrounding cells in the TME to exert pro-metastatic effects, or act in an endocrine manner through the bloodstream. For example, EVs from cells of the TME can increase the motility and invasive capacity of cancer cells, allowing them to invade local tissue and intravasate into the bloodstream for transport to distant parts of the body. Through the endocrine pathway, EVs also play a role in preparing the premetastatic niche in a distant secondary site to be a supportive environment for metastatic cancer cells.
2. Fibroblasts
Fibroblasts are the most abundant type of stromal cells and function to mediate tissue repair as well as maintain normal tissue homeostasis by producing and reorganizing different ECM proteins [16]. They remain dormant and non-proliferative in normal tissues and undergo an explosive expansion during the early phases of wound healing followed by massive apoptosis once the wound is repaired. Under tumorigenic conditions, fibroblasts are irreversibly activated and transition into cancer-associated fibroblasts (CAFs) that display more migratory behavior and vulnerability to epigenetic modifications [17]. Furthermore, CAF-derived EVs contain a variety of bioactive cargo that has been associated with regulating tumor development, metastasis, and therapeutic resistance [18] (Figure 2).
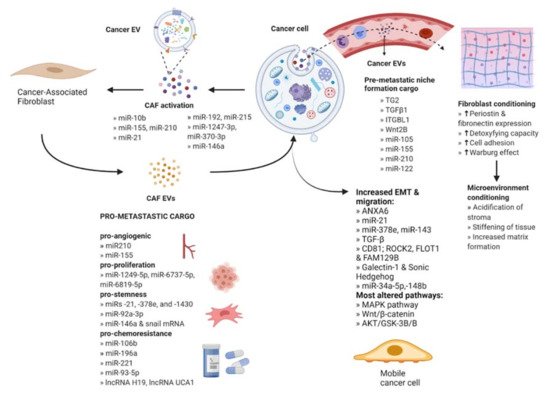
Figure 2. Intercellular communication between cancer cells and stromal fibroblasts. Cancer-derived EVs act on fibroblasts to facilitate activation into cancer-associated fibroblasts (CAFs). CAF EVs act on cancer cells to enhance their metastatic potential by delivering bioactive molecules. The released cargo modifies several key signaling pathways that promote angiogenesis, proliferation, stemness, chemoresistance, and EMT. Cancer EVs also act at a secondary site via the bloodstream through activation of local fibroblasts to CAFs which condition the microenvironment for the establishment of the pre-metastatic niche. An increase in the expression levels of proteins or mechanisms activity is indicated by upward arrows.
3. Endothelial Cells
The endothelium is a single layer of endothelial cells (EC) that lines the interior surface of all vessels in the cardiovascular and lymphatic systems and acts as an interface between the circulation and surrounding tissue. In non-cancer conditions, ECs and signals from ECs are crucial in the organization of the growth and development of connective tissues. ECs carry out myriads of tissue and organ-specific functions due to their capacity to adapt to their microenvironment [74]. They also elicit various responses from target cells by secreting EVs directly into the circulation. Interactions between EV surface proteins and the membrane receptors of the ECs then mediate EV uptake [75]. Phage display analysis has shown that ECs of the endothelium express various surface receptors depending on their location and functional status [76]. Therefore, ECs are important mediators of receptor-specific EV uptake in the endothelium and have been implicated in a variety of metastatic mechanisms (Figure 3).
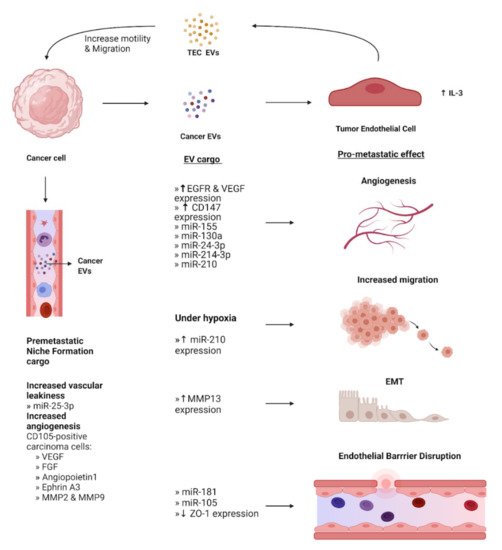
Figure 3. EV-Mediated crosstalk between cancer cells and endothelial cells. EVs containing cargo from cancer cells have various effects on the phenotype of surrounding endothelial cells, converting them into tumor endothelial cells (TECs). These effects include promoting angiogenesis, EMT, and increased migration under hypoxia. Additionally, cancer EVs mediate disruption of the endothelial barrier to aid the intravasation of cancer cells. EVs derived from TECs act on cancer cells to increase their motility and facilitate migration. Concurrently, cancer cells secrete EVs that travel to a secondary site and stimulate local endothelial cells that assist in the formation of the pre-metastatic niche. Expression levels of EV cargo are indicated by upward and downward arrows to represent increase and decrease, respectively.
4. Immune Cells
Immune cells in the TME include macrophages, dendritic cells, lymphocytes, neutrophils, myeloid-derived suppressor cells, and natural killer cells [103]. Many of these cells play an EV-mediated role in primary tumor progression, for example, by assisting in the escape of the tumor from immunosurveillance [104]. Tumor-associated macrophages (TAMs) are the main immune cell type infiltrating the TME and have been shown to engage in bidirectional EV-mediated cellular communication with tumor cells to promote metastasis [105] (Figure 4).
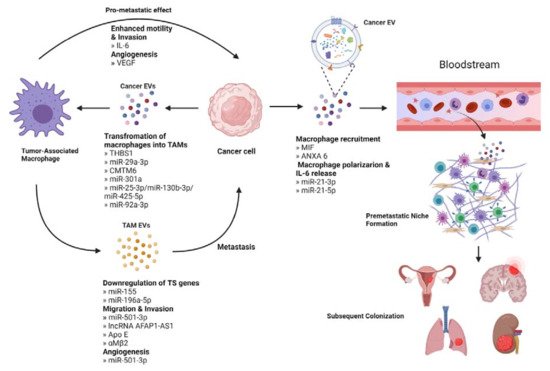
Figure 4. Interaction between cancer cells and tumor-associated macrophage (TAMs). Cancer derived EVs deliver factors like miRNAs that promote the transformation of macrophages into TAMs. TAMs can stimulate metastasis directly or through EV-mediated downregulation of tumor suppressor genes, increased migration, and invasion potential, and supporting angiogenesis. Cancer cells also release EVs that act in an endocrine manner to stimulate the recruitment of macrophages in a secondary site. These macrophages then undergo polarization to support the establishment of the pre-metastatic niche, aid to evade immune surveillance and contribute to subsequent colonization.
5. Potential Applications of EVs in Clinical Management and Treatment
The development of effective cancer treatments is hindered by tumor heterogeneity, and the complexity within the TME. Given the pivotal role of EVs in mediating intercellular communication, these factors represent a potential target for novel treatment approaches.
One application of cancer-derived EVs is as a biomarker for earlier diagnosis or treatment response predictions (Table 3 and Table 4). In particular, blood-based EVs and their miRNA cargo, which can be acquired and analyzed by minimally invasive techniques, have garnered considerable attention. For instance, EV integrins extracted from blood plasma have been shown to predict organ-specific metastasis from patients with breast and pancreatic cancer [129]. Additionally, though there has been an increase in overall survival for cohorts of metastatic colorectal cancer patients receiving targeted therapies, only a subset of patients respond favorably to such treatment. Recent expression analysis for a panel of miRNA species encapsulated in EVs successfully predicted the prognosis of metastatic CRC patients following first-line treatment with anti-angiogenic therapy [130]. EV-derived miR-21, a miRNA previously established with an oncogenic role in several cancers, from plasma has been also reported as a potential marker for recurrence in CRC. Its expression level was also significantly associated with the presence of liver metastases [131]. Furthermore, another study examined miRNA profiles of EVs secreted by primary and metastatic CRC cell lines that displayed different metastasis potentials. They revealed that exosomal miR-17-5p and miR-92a-3p expression were consistent with CRC progression [131]. Analysis of the two miRNAs on clinical serum samples showed that miR-17-5p was generally downregulated in CRC, but was expressed in higher levels from patients with distant metastases [131,132].
Table 3. Completed clinical trials utilizing extracellular vesicles as biological markers and the description of the circumstances under which they are used. Information from ClinicalTrials.gov, accessed on 1 November 2021.
| Study Type (Year) Estimated Enrollment |
Study Title | Type of Cancer | Description |
|---|---|---|---|
| NCT03262311 Clinical Trial (2021) 21 participants |
Pimo Study: Extracellular Vesicle-based Liquid Biopsy to Detect Hypoxia in Tumors | Invasive carcinomas: head and neck, lung, bladder, uterine cervix or breast | Hypoxia marker with prognostic and predictive value based on extracellular vesicles derived from blood samples to identify patients presenting tumor hypoxia that may benefit from sensitizer treatments or targeted radiotherapy. |
| NCT03228277 Clinical Trial Phase II (2017) 25 participants |
Olmutinib Trial in T790M (+) NSCLC Patients Detected by Liquid Biopsy Using BALF Extracellular Vesicular DNA | Non-Small Cell Lung Cancer (NSCLC) | Assess the anti-tumor efficacy of Olmutinib (Olita®) administered to patients with T790M-positive NSCLC by extraction of DNA from extracellular vesicles of bronchoalveolar lavage fluid. |
| NCT02662621 Clinical Trial (2015) 71 participants |
Pilot Study with the Aim to Quantify a Stress Protein in the Blood and in the Urine for the Monitoring and Early Diagnosis of Malignant Solid Tumors | Solid Tumors | Determine the utility of the stress protein HSP70, located at the membrane of EVs coming from cancer cells, as a marker for early diagnosis in blood and urine samples. |
| NCT04913545 Observational (2019) 18 participants |
The Sensitivity and Specificity of Using Salivary miRNAs in Detection of Malignant Transformation of Oral Lesions | Oral Premalignant Lesions | Evaluate the diagnostic accuracy of salivary extracellular vesicles miRNAs to detect the malignant transformation of the premalignant lesion. |
Table 4. Ongoing clinical trials registered in ClinicalTrials.gov (accessed on 1 November 2021) utilizing extracellular vesicles as biological indicators and the description of the circumstances under which they are implemented.
| Study Type (Year) Estimated Enrollment |
Study Title | Type of Cancer | Description |
|---|---|---|---|
| NCT04523389 Observational (2020) 172 participants |
Contents of Circulating Extracellular Vesicles: Biomarkers in Colorectal Cancer Patients | Colorectal Cancer | Study the potential of miRNAs contained within exosomes derived from tumors as biomarkers of early prognosis from blood samples. |
| NCT04852653 Observational (2021) 40 participants |
A Prospective Feasibility Study Evaluating Extracellular Vesicles Obtained by Liquid Biopsy for Neoadjuvant Treatment Response Assessment in Rectal Cancer | Rectal Cancer | Evaluate if the detection of tumor EVs from blood samples is a reliable biomarker for the differentiation of good responders to neoadjuvant chemoradiotherapy (nCRT). This will aid in the accurate identification of good responders to nCRT and spare them of the functional cost of total mesorectum excision. |
| NCT04742608 Observational (2020) 250 participants |
Development of Liquid Biopsy Technologies for Noninvasive Cancer Diagnostics in Patients with Suspicious Thyroid Nodules or Thyroid Cancer | * Thyroid Gland Carcinoma * Thyroid Gland Nodule |
Collection of blood and tissue samples from surgical resections of the thyroid. Posterior isolation and characterization of EVs, then perform an RNA and DNA panel to have a molecular profile to be used as a predictor for thyroid nodules or thyroid cancer. |
| NCT04164134 Observational (2018) 396 participants |
New Strategies to Detect Cancers in Carriers of Mutations in RB1 | Retinoblasto-ma (RB) | Development of non-invasive cancer test using blood samples for the detection of tumors through their derived EVs in RB1-mutation carriers, complemented with family cancer history. |
| NCT03957252 Observational (2019) 2800 participants |
Validation of Clarity DX Prostate as a Reflex Test to Refine the Prediction of Clinically-significant Prostate Cancer | Prostate Cancer | Determine the accuracy of the blood test Clarity DX as a reflex to PSA by extracellular vesicle profiling on patients suspected of prostate cancer who will undergo biopsy. Results will be compared to assess predictive accuracy. |
| NCT04529915 Observational (2020) 470 participants |
Multicenter Clinical Research for Early Diagnosis of Lung Cancer Using Blood Plasma Derived Exosome | Lung Cancer | Evaluate the possibility of distinguishing between normal and lung cancer patients through deep-learning analysis of blood abundant exosomes and the analysis of lung cancer specific exosomal protein. |
| NCT04638049 Interventional-Clinical Trial (2020) 50 participants |
Intestinal Microbiota in Prostate Cancer Patients as a Biomarker for Radiation-Induced Toxicity | * Prostate Cancer * Prostate Adenocarcinoma * Prostatic Neoplasms |
Examination of the microbiota composition (feces), the associated metabolome (blood, feces and urine) and bacterial extracellular vesicles (BEVs) (blood and feces) to establish a prospective biomarker in the pathophysiology of radiation-induced GI toxicity. |
| NCT04993378 Observational (2018) 40 participants |
Prospectively Predict the Efficacy of Treatment of Gastrointestinal Tumors Based on Peripheral Multi-omics Liquid Biopsy | Advanced Gastric Adenocarcinoma | To verify that four plasma EV-derived proteins generate a signature score that robustly predicts immunotherapeutic outcomes during different stages of the disease. |
| NCT02514681 Interventional-Clinical Trial (2015) 370 participants |
A Phase III Trial of Pertuzumab Retreatment in Previously Pertuzumab Treated Her2-Positive Advanced Breast Cancer | HER2-positive Locally Advanced or Metastatic Breast Cancer | Since Pertuzumab retreatment can be more effective than trastuzumab and chemotherapy-containing the study will evaluate its efficacy and safety. In addition, microRNA expression in extracellular vesicles after anti-HER2 therapy will be evaluated to find a prognostic and predictive biomarker. |
| NCT03576612 Interventional-Clinical Trial (2018) 36 participants |
GMCI, Nivolumab, and Radiation Therapy in Treating Patients with Newly Diagnosed High-Grade Gliomas | Glioma, Malignant | Assessment of safety, maximum tolerated dose and toxicity of combining GMCI plus nivolumab with standard of care radiation therapy, and temozolomide to treat patients with newly diagnosed high-grade gliomas. Determination of immune biomarkers including serum extracellular vesicles (EVs) based on surface and content proteins. |
| NCT04581382 Interventional-Clinical Trial (2020) 20 participants |
Radiation Therapy, Plasma Exchange, and Immunotherapy (Pembrolizumab or Nivolumab) for the Treatment of Melanoma | Melanoma | Establish the performance of radiation therapy, plasma exchange, and pembrolizumab or nivolumab. Association of the kinetics of extracellular vesicles after plasma exchange will be assessed with clinical outcome data. |
| NCT04298398 Interventional-Clinical Trial (2021) 108 participants |
Impact of Group Psychological Interventions on Extracellular Vesicles in People Who Had Cancer | Breast, prostate and colorectal cancer | Perform psychological interventions: Mindfulness-Based Cognitive Therapy (MBCT) and Emotion Focused Therapy for Cancer Recovery (EFT-CR) and explore any effect on extracellular vesicles and on psychological outcomes of people who had cancer. |
*: Thyroid gland carcinoma is a cancer of the thyroid, a small gland at the base of the neck that produces hormones. Thyroid nodules are solid or fluid-filled lumps formed within the thyroid, a small proportion of which can be cancerous. *: Prostate cancer, or prostate adenocarcinoma, occurs in the prostate, a gland in the pelvis and part of the male reproductive system.
This entry is adapted from the peer-reviewed paper 10.3390/cells10123429
This entry is offline, you can click here to edit this entry!
