Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Koelreuteria paniculata Laxm. is used in traditional medicine and has various established biological activities, however, the species is considered to be a potentially invasive alien tree species for Bulgarian flora. However, there is still much to be studied about the phytochemical and biological characteristics of the species.
- Koelreuteria paniculata
- dry ethanol extracts
- GC-MS analysis
- chemical compounds
- antitumor and antimicrobial activities
1. Introduction
Nowadays, more and more authors are studying the chemical composition of various plant extracts in order to find new sources, of plant origin, to combat resistant human pathogens and cancers. Emerging allergies and the many side effects of synthetic drugs are grounds to look for their natural alternatives that are sufficiently effective and, at the same time, less harmful to human health [1,2,3,4,5,6,7,8]. Antibiotic-resistant bacteria are a significant modern problem. The key to solving it can be individual plant compounds, essential oils, or extracts containing some of the most active phytochemicals with antibacterial activity, such as polyphenols and terpenes [8]. Many natural plant compounds also exhibit potent anti-cancer activity. The therapeutic value of herbal sources in the fight against cancer has increased in recent years worldwide, as evidenced by the use of certain chemotherapeutic drugs isolated from medicinal plants [6].
The present study was focused on the evaluation of the biologically active components of possible natural sources, such as Koelreuteria paniculata Laxm. (belonging to the family of Sapindaceae), known as the golden rain tree. The species is native to China, but it is widely used for ornamental purposes in Europe, including Bulgaria. It is now considered to be an invasive alien tree, characterized by good overall adaptability, growth, development, and strong vitality characteristics [9,10].
K. paniculata has been the subject of some phytochemical studies concerning various plant extracts, such as ethanol [2,11,12,13,14,15], methanol [1,13,14,15], benzene/ethanol [13,14,15], and formaldehyde [13], as solvents establishing the different groups of natural components. Phenolic derivatives based on gallic acid, catechins, and phenolic acids are isolated and determined from ethanol (vacuum) extracts of fresh leaf parts [11]. Flavonoids, phenolic acids, and sterols were examined from the ethyl acetate fraction of the K. paniculata flowers [16]. Primary (fatty acids, carbohydrates) and secondary metabolites (methyl gallate, ethyl gallate, flavonoids, and their glycosides, sterols, and saponins) are established from 70% ethanol extract of air-dried powdered aerial parts [2,12].
In a few studies, GC-MS analysis was performed in order to identify the chemical composition of the extracts [1,13,14,15]. The authors have analyzed several extracts of bark, wood, branches, leaves, and roots using GC-MS analysis; however, it was not implemented for the identification of the chemical composition of the plant flowers. They found different groups of active components (fatty acids, phenols, mono and triterpenes, sterols, vitamin E, and others). In a previous study by our team, four essential oils from the aerial parts of K. paniculata have been isolated by hydrodistillation, for the first time, and they were identified by GC-MS analysis [17]. We found a rich content of different classes of compounds, as follows: aliphatic oxygenated compounds, oxygenated sesquiterpenes, sesquiterpene hydrocarbons, aliphatic hydrocarbons, and diterpenes as predominant groups.
The biological activities exhibited by plants are due to their composition, certain groups of compounds, and the possible interactions between them. The fractions of methanol leaf extract in K. paniculata were distinguished by antibacterial and antifungal activity against Staphylococcus aureus, Bacillus subtilis, and Pyricularia grisea [1]. The ethanol extracts obtained from the K. paniculata aerial parts showed antibacterial (against E. coli) and antimalarial (against chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum) activities. Some compounds (such as methyl gallate and ethyl gallate) were among the biologically active secondary metabolites of the plant [2].
Galenic preparations of the K. paniculata leaf extracts exhibited high efficiency against six pathogenic microorganisms causing several diseases in humans, such as the following: Enterococcus faecalis, Proteus mirabilis, Seracia marcescens, Salmonella typhimurium, Campylobacter jejuni, and Escherichia coli [5].
The antitumor activity of K. paniculata extracts has been poorly studied. Kumar et al. [18] reported on the DNA protective effect of the methanol leaf extract and its hexane fraction. The antineoplastic activity of the carotenoid fraction of K. paniculata flowers was determined by Zhelev et al. [19]. The authors established low cytotoxicity against human hepatocarcinoma cell lines (HepG2) and human breast cancer cells (MDA-MB-231).
2. Chemical Compounds of Dry Ethanol Extracts from Aerial Parts of K. paniculata
The obtained dry (under vacuum) ethanol extracts’ yields were 0.6521 (2.6084) g for stem bark, 0.624 (2.4960) g for leaves, and 0.516 (2.0640) g for flowers. The extracts were viscous liquids with a dark brown color and characteristic ointment. The three samples were analyzed via GC-MS analysis. The chemical compounds (with their peaks) are presented in Table 1 and Figure 1.
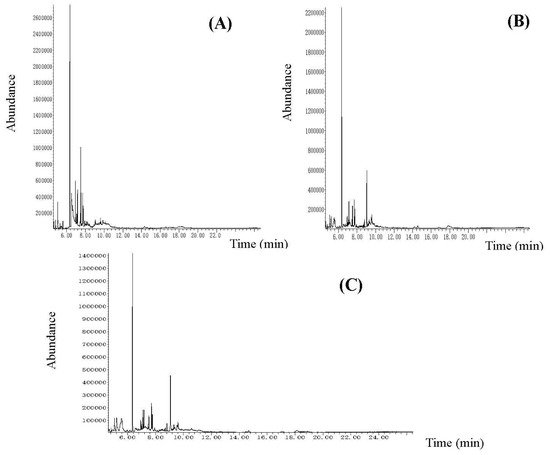
Figure 1. The GC-MS chromatograms of the analyzed dry ethanol extracts from K. paniculata: (A) flower extract, (B) leaf extract, (C) stem bark extract.
Table 1. Chemical compounds of the ethanol dry extracts of Koelreuteria paniculata aerial parts, (mean ± SD).
| Peak | RT | RIcalc | RIlit | Compound | K. paniculata, % of TIC | Compounds Identification |
||
|---|---|---|---|---|---|---|---|---|
| Flower | Leaf | Stem Bark |
||||||
| 1 | 4.49 | 1174 | 1170 | Furfuryl butanoate | nd | nd | nd | |
| 2 | 4.68 | 1190 | 1186 | (3Z)-Hexenyl butanoate | 0.81 ± 0.0 | 0.49 ± 0.0 | 0.97 ± 0.01 | GMD |
| 3 | 4.79 | 1197 | 1199 | γ-terpineol | 3.78 ± 0.10 | 0.85 ± 0.01 | 0.55 ± 0.0 | GMD |
| 4 | 5.10 | 1206 | 1202 | (2E,4E)-Hexadienol isobutanoate | 0.13 ± 0.0 | 2.00 ± 0.07 | 2.66 ± 0.08 | GMD |
| 5 | 5.20 | 1218 | 1217 | (2E,4E)-Nonadienol | 0.20 ± 0.0 | 3.53 ± 0.09 | 4.68 ± 0.11 | GMD |
| 6 | 5.26 | 1225 | 1223 | Methyl nonanoate | 0.58 ± 0.0 | 0.22 ± 0.0 | 0.44 ± 0.0 | GMD |
| 7 | 5.30 | 1233 | 1228 | Nerol | nd | 0.49 ± 0.0 | 1.71 ± 0.05 | GMD |
| 8 | 5.41 | 1231 | 1229 | (3Z)-Hexenyl 2-methyl butanoate | 0.36 ± 0.0 | 3.78 ± 0.10 | 8.15 ± 0.20 | GMD |
| 9 | 5.60 | 1288 | 1288 | Lavandulol acetate | 0.47 ± 0.01 | 3.40 ± 0.09 | 2.02 ± 0.06 | GMD |
| 10 | 6.11 | 1316 | 1315 | δ-terpinyl acetate | 0.12 ± 0.0 | 0.71 ± 0.0 | 0.93 ± 0.01 | GMD |
| 11 | 6.23 | 1345 | 1346 | α-terpinyl acetate | 16.42 ± 0.51 | 20.24 ± 0.07 | 3.56 ± 0.09 | GMD |
| 12 | 6.35 | 1359 | 1360 | Neryl acetate | 0.15 ± 0.0 | 0.33 ± 0.0 | 12.37 ± 0.40 | GMD |
| 13 | 6.40 | 1362 | 1361 | Pyrogallol | 20.86 ± 0.70 | 2.02 ± 0.07 | 1.91 ± 0.05 | NIST’08 |
| 14 | 6.55 | 1366 | 1363 | α-methyl benzyl butyrate | nd | 0.30 ± 0.0 | 0.82 ± 0.0 | GMD |
| 15 | 6.64 | 1375 | 1378 | (3Z)-Hexenyl hexanoate | nd | 0.79 ± 0.0 | 0.78 ± 0.0 | GMD |
| 16 | 6.82 | 1386 | 1386 | Isobutyl phenylacetate | nd | 1.13 ± 0.04 | 1.69 ± 0.04 | GMD |
| 17 | 6.94 | 1394 | 1395 | Ethyl decanoate | 5.85 ± 0.12 | 1.40 ± 0.05 | 1.47 ± 0.04 | GMD |
| 18 | 7.01 | 1401 | 1400 | n-tetradecane | 2.13 ± 0.07 | 1.08 ± 0.04 | 2.65 ± 0.07 | GMD |
| 19 | 7.08 | 1416 | 1417 | β-caryophyllene | 2.61 ± 0.07 | 3.86 ± 0.08 | 2.91 ± 0.07 | GMD |
| 20 | 7.12 | 1432 | 1433 | α-trans-bergamotene | 2.45 ± 0.07 | 0.95 ± 0.01 | 0.93 ± 0.01 | GMD |
| 21 | 7.16 | 1439 | 1438 | Phenyl ethyl butanoate | 3.89 ± 0.08 | 2.94 ± 0.08 | 2.89 ± 0.08 | GMD |
| 22 | 7.26 | 1453 | 1453 | Geranyl acetone | nd | 1.80± | 2.53 ± 0.07 | GMD |
| 23 | 7.41 | 1465 | 1466 | Linalool isovalerate | nd | 0.92 ± 0.01 | 1.42 ± 0.03 | GMD |
| 24 | 7.47 | 1469 | 1468 | n-dodecanol | 2.21 ± 0.07 | 0.99 ± 0.01 | 1.90 ± 0.04 | GMD |
| 25 | 7.51 | 1472 | 1471 | α-terpinyl isobutanoate | 10.32 ± 0.40 | 4.77 ± 0.10 | 3.56 ± 0.09 | GMD |
| 26 | 7.55 | 1476 | 1479 | α-curcumene | nd | 0.45 ± 0.0 | nd | GMD |
| 27 | 7.64 | 1481 | 1482 | γ-curcumene | nd | 0.58 ± 0.0 | nd | GMD |
| 28 | 7.72 | 1486 | 1485 | Phenyl ethyl 2-methylbutanoate | 0.75 ± 0.0 | 3.21 ± 0.08 | 3.84 ± 0.09 | GMD |
| 29 | 7.76 | 1488 | 1489 | β-selinene | 3.01 ± 0.07 | 2.60 ± 0.07 | 2.63 ± 0.07 | GMD |
| 30 | 7.97 | 1497 | 1498 | α-selinene | 3.36 ± 0.08 | 0.66 ± 0.0 | 0.72 ± 0.0 | GMD |
| 31 | 8.15 | 1511 | 1514 | β-curcumene | nd | 0.33 ± 0.0 | 0.15 ± 0.0 | GMD |
| 32 | 8.26 | 1556 | 1552 | Lauric acid | 1.93 ± 0.03 | 0.88 ± 0.0 | 0.36 ± 0.0 | NIST’08 |
| 33 | 8.41 | 1562 | 1560 | Geranyl butanoate | 0.81 ± 0.0 | 0.55 ± 0.0 | 0.78 ± 0.0 | GMD |
| 34 | 8.50 | 1568 | 1570 | Octyl hexanoate | 0.40 ± 0.0 | 0.62 ± 0.0 | 0.51 ± 0.0 | NIST’08 |
| 35 | 8.56 | 1576 | 1573 | Decyl butyrate | 0.64 ± 0.0 | 0.37 ± 0.0 | 0.44 ± 0.0 | NIST’08 |
| 36 | 8.71 | 1611 | 1611 | Tetradecanal | 0.20 ± 0.0 | 0.74 ± 0.0 | 0.82 ± 0.0 | GMD |
| 37 | 8.83 | 1623 | 1622 | Isobutyl cinnamate | nd | 1.22 ± 0.02 | 1.50 ± 0.02 | GMD |
| 38 | 9.07 | 1640 | 1639 | Phenyl ethyl hexanoate | 1.71 ± 0.03 | 9.05 ± 0.10 | 8.67 ± 0.07 | GMD |
| 39 | 9.17 | 1649 | 1650 | β-eudesmol | 0.42 ± 0.0 | 0.90 ± 0.01 | 0.69 ± 0.0 | GMD |
| 40 | 9.26 | 1654 | 1652 | α-eudesmol | 0.27 ± 0.0 | 0.72 ± 0.0 | 1.12 ± 0.02 | GMD |
| 41 | 9.37 | 1662 | 1661 | Dihydro-eudesmol | 0.35 ± 0.0 | 1.35 ± 0.02 | 1.93 ± 0.03 | GMD |
| 42 | 9.61 | 1671 | 1670 | Epi-β-bisabolol | 2.19 ± 0.07 | 3.03 ± 0.07 | 1.51 ± 0.02 | GMD |
| 43 | 9.67 | 1675 | 1674 | β-bisabolol | 2.88 ± 0.07 | 1.44 ± 0.02 | 1.66 ± 0.02 | GMD |
| 44 | 9.86 | 1690 | 1691 | (Z)-α-trans-Bergamotol | 2.00 ± 0.06 | 2.52 ± 0.07 | 1.00 ± 0.02 | GMD |
| 45 | 10.21 | 1699 | 1698 | (2Z,6Z)-Farnesol | 0.84 ± 0.0 | 0.51 ± 0.0 | 0.72 ± 0.0 | GMD |
| 46 | 10.50 | 1706 | 1704 | δ-dodecalactone | 0.97 ± 0.01 | 0.30 ± 0.0 | 0.57 ± 0.0 | GMD |
| 47 | 10.82 | 1718 | 1718 | Methyl eudesmate | nd | 0.45 ± 0.0 | 1.18 ± 0.02 | GMD |
| 48 | 11.93 | 1826 | 1825 | (E)-Nerolidyl isobutyrate | nd | 0.39 ± 0.0 | nd | GMD |
| 49 | 14.49 | 1953 | 1948 | Phytol | nd | nd | 0.14 ± 0.0 | GMD |
| 50 | 14.61 | 1971 | 1966 | Palmitic acid | 1.85 ± 0.05 | 0.86 ± 0.0 | 1.16 ± 0.02 | GMD |
| 51 | 14.73 | 1992 | 1990 | Ethyl palmitate | 0.29 ± 0.0 | nd | nd | GMD |
| 52 | 16.67 | 2018 | 2019 | (6E,10Z)-Pseudo phytol | nd | nd | 0.42 ± 0.0 | GMD |
| 53 | 16.85 | 2026 | 2024 | Isopropyl hexadecanoate | nd | 0.79 ± 0.0 | 0.25 ± 0.0 | GMD |
| 54 | 16.94 | 2029 | 2030 | (6E,10E)-Pseudo phytol | nd | nd | 0.81 ± 0.0 | GMD |
| 55 | 18.05 | 2133 | 2132 | Linoleic acid | 0.16 ± 0.0 | 4.32 ± 0.04 | 1.55 ± 0.02 | GMD |
| 56 | 18.28 | 2142 | 2141 | Oleic acid | 0.23 ± 0.0 | nd | nd | GMD |
| 57 | 21.58 | 2400 | 2400 | Tetracosane | 0.10 ± 0.0 | nd | nd | GMD |
| Total identified, % | 98.70 | 97.83 | 98.63 | |||||
RT—Retention time; RIcalc—Kovats retention index, calculated by authors; RIlit—Kovats retention index by literature data; TIC—Total ion current; nd—not detected; NIST’08—National Institute of Standards and Technology, Gaithersburg, MD, USA; GMD—Golm Metabolome Database.
Forty components were identified in the flower extract, representing 98.70% of the total content. Eighteen of them were in a concentration above 1%. The main components (over 3%) were as follows: pyrogallol (20.86%), α-terpinyl acetate (16.42%), α-terpinyl isobutanoate (10.32%), ethyl decanoate (5.85%), phenyl ethyl butanoate (3.89%), γ-terpineol (3.78%), α-selinene (3.36%), and β-selinene (3.01%). Qu et al. [16] first identified, in K. paniculata flowers, nine components in the ethyl acetate fraction by column chromatography and spectral analysis. The components are the following: sitosterol glucoside, gallic acid, kaempferol, luteolin, kaempferol-3-O-(6″-acetyl)-β-d-glucopyranoside, hyperoside-2″-O-acetyl, hyperoside-2″-O-galloyl, hyperoside, and kaempferol-3-O-d-glucopyranoside. In our previous study related to the essential oil composition of K. paniculata flowers, there were 38 phytochemicals identified, with twelve main compounds. The five common components for the two types of extracts have been proven, namely the following: β-caryophyllene, lauric acid, palmitic acid, oleic acid, and tetracosane [17].
In the leaf extract, 50 components were identified, which represents 97.83% of the total content. Twenty-two of them were found in a concentration above 1% and the major ones (over 3%) were the following ten: α-terpinyl acetate (20.24%), phenyl ethyl hexanoate (9.05%), α-terpinyl isobutanoate (4.77%), linoleic acid (4.32%), β-caryophyllene (3.86%), (3Z)-hexenyl 2-methyl butanoate (3.78%), (2E,4E)-nonadienol (3,53%), lavandulol acetate (3.40%), phenyl ethyl 2-methylbutanoate (3.21%), and epi-β-bisabolol (3.03%). In the analyzed fractions of methanol extract from the dry leaves of K. paniculata, Ghahari et al. [1] found a smaller number (between two and seven) of the major components (over 3%), of which only linoleic acid (4.69%) was among those found by us. Wang et al. [14] isolated 13 active substances in both ethanol and methanol leaf extracts and 32 in benzene/ethanol leaf extract in which the best represented is ethyl gallate—a phenolic compound with antitumor activity. Andonova et al. [17] identified 49 components in the leaf essential oil from golden rain trees, of which six of them are the major ones (above 3%), different from those in the ethanol leaf extract. Only palmitic acid (2.89%), lauric acid (<1%), and β-caryophyllene (<1%) are common.
Fifty components were identified in the bark extract, representing 98.63% of the total content, and twenty-nine of them were in concentrations above 1%. The main components (over 3%) were as follows: neryl acetate (12.37%), (3Z)-hexenyl 2-methyl butanoate (8.15%), (2E, 4E)-nonadienol (4.68%), phenyl ethyl 2-methylbutanoate (3.84%), and α-terpinyl acetate (3.56%). Yang et al. [13] analyzed the ethanol bark extract of K. paniculata using GC-MS analysis and identified the components palmitic acid, linoleic acid, and ethyl oleate. The first two were also present in our extract in similar concentrations. The same authors reported data on the components of other types of bark extracts (formaldehyde, phenyl alcohol, and benzene alcohol extracts), where the concentrations of palmitic acid methyl ester (1.21%), vitamin E (0.31%), sorbitol (0.25%), and dihydrojasmone (0.18%) were identified. The authors found that the main components of the bark extracts were oleic acid (38.72%), lauric acid (5.90%), and acetic acid (4.41%), which were identified by TD-GC-MS analysis. GC-MS analysis of the bark essential oil of the golden rain tree conducted by Andonova et al. [17] identified thirty-six components with nine major ones. Six compounds of all identified were comparable with those in the ethanol extract-palmitic acid (3.20%), β-caryophyllene (1.81%), phytol (1.80%), oleic acid (1.03%), lauric acid (<1%), and tetracosane (<1%).
A comparative analysis of the chemical composition of the studied extracts showed that the one obtained from the flowers was dominated by pyrogallol. It is an odorless substituent and does not form the odor of the extract but instead determines its biological properties, mainly the antioxidant and antimicrobial potential [20]. The extracts of the flowers were also dominated by the monoterpene alcohol γ-terpineol, as well as its esters with acetic and isobutyric acid, which forms the smell of the extract as fresh bergamot-lavender-like (terpinyl acetate), floral (terpinyl isobutyrate), and pine with floral notes (γ-terpineol). According to our findings, the amount of these compounds was lower in the extracts obtained by the plant leaves and bark.
The differences in the identified components in our study, compared with those reported in the literature, are due to the plant’s growing conditions, the technological parameters of the extraction, and the specificity of the used methodology.
The distribution of the components by chemical groups is presented in Figure 2. Oxygenated monoterpene (OM) derivatives predominated in all three of the extracts (flowers 32.49 ± 0.30%, leaves 35.21 ± 0.30% and stem bark 29.84 ± 0.25%), followed by aliphatic oxygen (AO) derivatives and phenylpropanoids (PP). The other groups were less represented, and their distribution can be seen in the figure, as the deviation of the values ranged from 0.07 to 0.1 for sesquiterpene hydrocarbons, from 0.15 to 0.20 for oxygenated aliphatics, 0.01 for aliphatic hydrocarbons, 0.08 to 0.09 for oxygenated sesquiterpenes, and from 0.20 to 0.26 for phenylpropanoids.
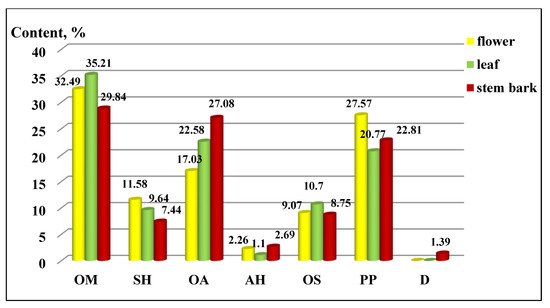
Figure 2. Composition by chemical groups from aerial parts in Koelreuteria paniculata ethanol extracts (%): AH—Aliphatic hydrocarbons; OA—Oxygenated aliphatics; OM—Oxygenated monoterpenes; SH—Sesquiterpene hydrocarbons; OS—oxygenated sesquiterpenes; PP—Phenylpropanoids; D—Diterpenes.
In our previous study, the distribution of the components in the different parts of K. paniculata showed some differences as aliphatic and oxygenated hydrocarbons, and sesquiterpenes represented the main part of the isolated essential oils [17].
The distribution of the functional groups concerning the total percentage content of K. paniculata ethanol extracts is presented in Figure 3. The group of esters had the highest percentage in all three of the studied extracts, followed by alcohols. An exception was the high content of phenols in the flowers (21.13 ± 0.20%), compared to the other two plant parts, which were present in a low percentage. The groups of acids, phenols, ketones, lactones, and aldehydes were very poorly represented in the examined extracts, as shown in Figure 3. The arrangement of the compounds by the functional groups was related to the manifested biological activities of the extracts. Gabrielli et al. [21] pointed out that phenols, followed by alcohols, aldehydes, ketones, ethers, and hydrocarbons, were of primary importance for the activity of the essential oils.
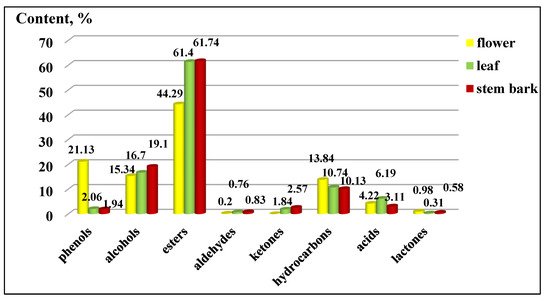
Figure 3. Composition by functional groups from Koelreuteria paniculata ethanol extracts (%): phenols; alcohols; esters; aldehydes; ketones; hydrocarbons, acids; lactones. The deviations in the values are in the range of 0.5 to 0.9% statistical error.
3. Antitumor Activity of the K. paniculata Ethanol Dry Extracts
The antiproliferative activity of the K. paniculata ethanol extracts obtained from the different plant parts was examined on two tumor cell lines—HT-29 and PC3. The two cell lines were not randomly selected. The human colon adenocarcinoma HT-29 cell line is widely used to study the biology of human colon cancers and showed many characteristics of mature intestinal cells [22]. Another cell line, PC3, is also valuable in carcinogenesis. Prostate cancer is the primary malignancy in men and the second leading cause of cancer-related deaths [23]. The obtained results are shown in Figure 4 and Table 2. Improved antiproliferative activity of the flower extract over the other two extracts (IC50-21.44 µg/mL) on the cell line HT-29 was observed. Less pronounced activity (over two times) on the other cell line PC3 (IC50-58.76 µg/mL) was also demonstrated. The leaf extract showed almost the same activity as the flower extract on the HT-29 cell line (IC50-23.63 µg/mL), while prostate cancer cells were less sensitive to this extract (IC50–80.56 µg/mL). The bark extract showed weak inhibition effects on the cell lines (IC50–339.4 µg/mL and 182.8 µg/mL for HT-29 and PC3 cell lines, respectively). As can be seen from the graphs (Figure 4E,F), the antiproliferative activity of the total bark extract was dose-dependent for both cell lines. It strongly resembled the antiproliferative effect of cisplatin, the antitumor standard in the present study. The total leaf and flower extracts affected cell growth only at low concentrations, and had almost the same values at higher concentrations at over 60 mg/mL for the HT-29 (Figure 4A,C) and over 125 mg/mL for PC3 (Figure 4B,D). As a possible reason for this, we can point out the differences in the chemical composition of the plant parts and the ethanol extracts obtained from them, especially the presence of the high content of pyrogallol in the composition of flowers. Pyrogallol is compared to antibiotics and also has antioxidant properties [20]. For example, Ahn et al. [24] reported the antitumor mechanisms of pyrogallol that showed significant cytotoxicity and reduced the number of colonies in Hep3B and Huh7 cells. Other authors revealed that phenols determined the antitumor effect of plant extracts on various tumor cell lines (including HT-29) [25,26].
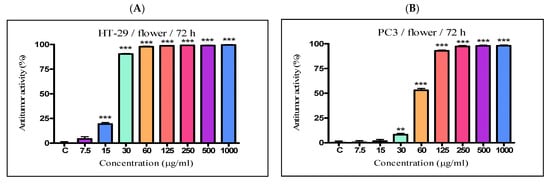
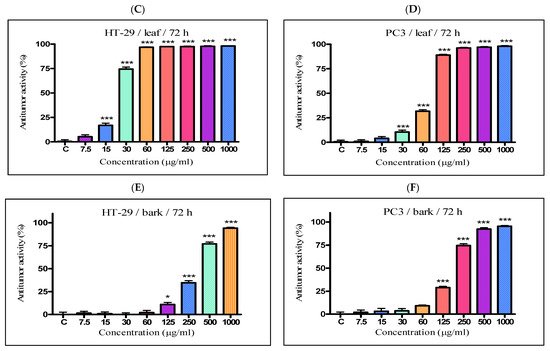
Figure 4. In vitro antiproliferative activity of ethanol extracts from three plant parts of K. paniculata. MTT assay was performed after 72 h. (A,C,E) show data for antiproliferative activity on cell line HT-29, of flower, leaf, and bark, respectively. (B,D,F) indicate results obtained on cell line PC3, of the same plant parts respectively. All samples were analyzed in triplicates. Values are represented as mean ± SD; One-way ANOVA followed by post hoc test using Tukey’s multi-group comparison was performed: * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 2. In vitro antiproliferative activity of the ethanol dry extracts of Koelreuteria paniculata aerial parts.
| IC50 of Mean ± SD (µg/mL) | ||
|---|---|---|
| HT-29 | PC3 | |
| Flower extract | 21.44 ± 0.20 | 58.76 ± 0.53 |
| Leaf extract | 23.63 ± 0.22 | 80.56 ± 0.75 |
| Stem Bark extract | 339.4 ± 1.31 | 182.8 ± 1.65 |
| Cisplatin | 2.5 ± 0.08 | 1.01 ± 0.03 |
IC50 determined following 72 h treatment with ethanol extracts. Antitumor activities were expressed as IC50 values (extract concentrations (µg/mL) required for 50% inhibition of cell growth), calculated using non-linear regression analysis (GraphPad Software, San Diego, CA, USA). Results were calculated from three measurements and expressed as mean ± SD. Cisplatin was used as standard to confirm the suitability of the used antitumor method.
Compared to the findings in our study, Zhelev et al. [19], using the MTT-test, found that the carotenoid fraction from K. paniculata flowers demonstrate relatively low cytotoxicity to HepG2 (human hepatocarcinoma) and MDA-MB-231 (human breast cancer cells), such as the HepG2 cell line, is more sensitive. The research, in this case, was related to the cytotoxicity of carotenoids and did not investigate their antiproliferative activity. Several articles have examined the ability of different K. paniculata extracts to protect various DNA structures from damaging factors. In the study by Kumar et al. [18,27], the methanol extracts and different fractions from the leaves showed a DNA protective effect in Calf thymus/pUC18, as authors associated its activity with the polyphenol constituents within it. In addition, Kumar and Kaur [28] established the potential of those extracts to inhibit lipid peroxidation and 4-nitroquinoline-1-oxide (4NQO)-induced genotoxicity. In vitro cytotoxicity assay on another Koelreuteria species (K. elegans) showed the promising anticancer activity of two phenols (from butanol fraction), methyl gallate and austrobailignan, against MCF-7 cell lines, which also reduced the cell proliferation of it [29].
4. Antimicrobial Activity of the K. paniculata Ethanol Dry Extracts
The results for the tested amounts of the extracts (100 μL, 150 μL) on nine pathogenic strains of microorganisms are presented in Table 3 and Figure 5. The bark extract was the most effective against the Gram-positive bacteria Bacillus subtilis ATCC 6633 (18 mm inhibition zone, IZ), Bacillus cereus NCTC 10,320 (14 mm IZ), and against the Gram-negative bacteria Pseudomonas aeruginosa ATCC 6027 (14 mm IZ) and Proteus vulgaris ATCC 6380 (8 mm IZ) at the higher tested concentration of the extract. The inhibitory zone of K. paniculata flower extract was quite similar against P. vulgaris (10 mm IZ), B. subtilis (14 mm IZ), and B. cereus (14 mm IZ). On the other hand, the K. paniculata leaf extract did not inhibit the test cultures against the Gram-negative bacterium E. coli ATCC 8739.
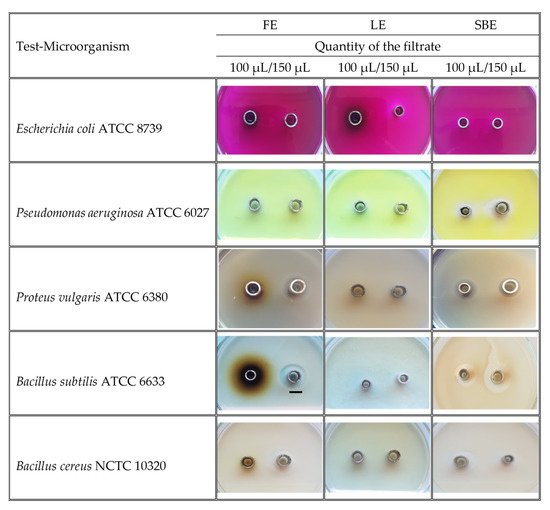
Figure 5. Images of the growth inhibition zones (over 6 mm) of dry ethanol extracts of Koelreuteria paniculata aerial parts; FE—Flower extract; LE—Leaf extract; SBE—Stem Bark extract. Scale bar indicated 10 mm.
Table 3. Zones of growth inhibition (mm) of dry ethanol extracts of Koelreuteria paniculata aerial parts.
| Test-Microorganism | QF | FE | LE | SBE | CH |
|---|---|---|---|---|---|
| Gram-negative bacteria | |||||
| Escherichia coli ATCC 8739 |
150 μL | - * | 9.02 a ± 0.01 | - | 28.05 b ± 0.01 |
| 100 μL | - | 6.02 a ± 0.01 | - | ||
| Salmonella enterica NCTC 6017 | 150 μL | - | - | - | 24.04 ± 0.03 |
| 100 μL | - | - | - | ||
| Klebsiella (clinical isolate) |
150 μL | - | - | - | 21.03 ± 0.03 |
| 100 μL | - | - | - | ||
| Pseudomonas aeruginosa ATCC 6027 | 150 μL | - | - | 14.02 c ± 0.01 | 21.03 c ± 0.03 |
| 100 μL | - | - | 5.03 c ± 0.01 | ||
| Proteus vulgaris ATCC 6380 | 150 μL | 10.04 d ± 0.02 | - | 8.04 d ± 0.01 | - |
| 100 μL | 6.05 d ± 0.04 | - | 6.02 d ± 0.01 | ||
| Gram-positive bacteria | |||||
| Staphylococcus aureus ATCC 25093 |
150 μL | - | - | - | 25.03 ± 0.03 |
| 100 μL | - | - | - | ||
| Bacillus subtilis ATCC 6633 |
150 μL | 14.03 e ± 0.03 | - | 18.04 e ± 0.02 | 39.04 f ± 0.03 |
| 100 μL | 11.03 e ± 0.04 | - | 11.03 e ± 0.02 | ||
| Bacillus cereus NCTC 10320 | 150 μL | 14.0 6 g ± 0.03 | - | 14.03 g ± 0.02 | - |
| 100 μL | - | - | 6.02 g ± 0.01 | ||
| Listeria monocytogenes NCTC 11994 |
150 μL | - | - | - | 21.06 ± 0.02 |
| 100 μL | - | - | - | ||
Data are presented as means ± SD (standard deviation). a–g Means in a row not sharing the same superscript letter are significantly different at p < 0.05 (Tukey’s HSD test). QF—Quantity of the filtrate; FE—Flower extract; LE—Leaf extract; SBE—Stem bark extract; CH—Chlorhexidine; *—No inhibitory activity was observed.
The differences in IZ values could be explained by the content of pyrogallol and terpineol esters. It is known that the activity on the main components of aromatic products (essential oils, extracts) was arranged in the following sequence: phenols > alcohols > aldehydes > ketones > esthers > hydrocarbons [30].
There was limited information concerning the antimicrobial properties of K. paniculata, as the reports were mainly about extract obtained from the plant’s leaves. This is the first paper studying the antibacterial activity of extracts obtained from K. paniculata flowers and stem barks. Ghahari et al. [1] reported the antibacterial activity of K. paniculata methanol extract from the leaves against B. subtilis and S. aureus. Zazharskyi et al. [5] investigated the antimicrobial potential (with inhibition zone above 8 mm) of ethanol extracts from golden rain tree extracts against different pathogens, such as the following: E. faecalis, P. mirabilis, S. marcescens, S. typhimurium, C. jejuni, and E. coli; the last of which was the most sensitive microorganism. The authors did not find activity against the tested P. aeruginosa compared to the findings reported in our study. Ethyl and methyl gallate were the investigated phenols demonstrated in the study by Mostafa et al. [2]. They were reported as promising antimicrobial (against E. coli) and antimalarial (against chloroquine-sensitive plasmodia-Plasmodium falciparum) agents.
The antimicrobial activity of different plants is influenced by the chemical composition of the plant and the concentration and conditions of obtaining the extracts. For example, Ham et al. [31] reported that neryl acetate had significantly strong and selective antibacterial activity against Gram-negative fish pathogens. Therefore, the presence of the component in the stem bark extracts could be the reason for its antimicrobial potential. Another study revealed the α-terpinyl acetate essential oil and extracts showed high antimicrobial effect against fungi, dermatophytes, bacteria and Candida yeasts [32]. The strain differences between the test cultures may also be relevant to the reported results [33].
This entry is adapted from the peer-reviewed paper 10.3390/plants10122715
This entry is offline, you can click here to edit this entry!
